Amivantamab plus Chemotherapy in NSCLC with EGFR Exon 20 Insertions
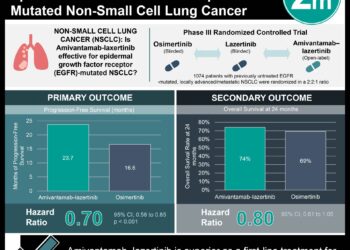
1. Median progression-free survival was 11.4 months in the amivantamab–chemotherapy group and 6.7 months in the chemotherapy group with HR 0.40.
2. Serious adverse events occurred in 37% of the amivantamab–chemotherapy group and 31% of the chemotherapy group.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Exon 20 insertions are the third most common mutation in epidermal growth factor receptor (EGFR) mutations in non-small-cell lung cancer (NSCLC). Currently, platinum-based chemotherapy is the first-line therapy for these patients, but it has limited efficacy, and tyrosine kinase inhibitors are largely ineffective with this mutation. Amivantamab, a bispecific antibody, has shown promise in early trials for EGFR exon 20 insertion NSCLC. This study assessed the effectiveness and safety of amivantamab combined with chemotherapy compared to standard chemotherapy alone as the first-line treatment for advanced NSCLC with EGFR exon 20 insertions. The primary outcome was progression-free survival (PFS) and secondary outcomes included objective response rate (ORR), overall survival (OS), duration of response (DoR), and safety. Median PFS was 11.4 months in the amivantamab–chemotherapy group and 6.7 months in the chemotherapy group with HR 0.40 (p<0.001). At 18 months, PFS was 31% vs 3% respectively. ORR (complete or partial response) was 73% in the amivantamab–chemotherapy group and 47% in the chemotherapy group, rate ratio of 1.50 (p<0.001). The median DoR was 9.7 months with amivantamab–chemotherapy and 4.4 months with chemotherapy; and the median time until response was 6.7 weeks and 11.4 weeks respectively. OS was not mature at the time of analysis. Serious adverse events occurred in 37% of the amivantamab–chemotherapy group and 31% of the chemotherapy group, with the most common grade ≥3 adverse events being neutropenia (in 33%), leukopenia (11%), and rash (in 11%) with amivantamab–chemotherapy and neutropenia (in 23%), anemia (in 12%), and thrombocytopenia (in 10%) with chemotherapy. Infusion-related reactions occurred in 42% vs 1% respectively. The strengths of this study included its methodology and the limitations included immature data (OS) and a small number of patients. Overall, this study found the combination of amivantamab and chemotherapy compared to chemotherapy alone showed improvement in outcome measures in previously untreated, advanced NSCLC with EGFR exon 20 insertions.
Click to read the study in NEJM
In-Depth [randomized controlled trial]: This phase 3, international trial randomized (1:1) adults with no previous treatment for advanced NSCLC with EGFR exam 20 mutations into 2 groups; amivantamab–chemotherapy (153 patients) or chemotherapy alone (155). Chemotherapy consisted of carboplatin and pemetrexed. With a median follow-up of 14.9 months, the median treatment duration was 9.7 months (0.1 to 26.9) with amivantamab–chemotherapy and 6.7 months (0 to 25.3) with chemotherapy. Median PFS was 11.4 months (95%CI, 9.8-13.7) in the amivantamab–chemotherapy group and 6.7 months (95%CI, 5.6-7.3) in the chemotherapy group with HR 0.40 (95%CI, 0.30-0.53, p<0.001). At 18 months, PFS was 31% vs 3% respectively. ORR (complete or partial response) was 73% (95%CI, 65-80) in the amivantamab–chemotherapy group and 47% (95%CI, 39-56) in the chemotherapy group, rate ratio 1.50 (95%CI, 1.32-1.68, p<0.001). Median DoR was 9.7 months (95%CI, 8.2-13.5) with amivantamab–chemotherapy and 4.4 months (95%CI, 4.1-5.6) with chemotherapy; and the median time until response was 6.7 weeks (5.1-72.5) and 11.4 weeks (5.1-60.2) respectively. OS was not mature at the time of analysis. Serious adverse events occurred in 37% of the amivantamab–chemotherapy group and 31% of the chemotherapy group, with the most common grade ≥3 adverse events being neutropenia (in 33%), leukopenia (11%), and rash (in 11%) with amivantamab–chemotherapy and neutropenia (in 23%), anemia (in 12%), and thrombocytopenia (in 10%) with chemotherapy. Infusion-related reactions occurred in 42% vs 1% respectively. Overall, this study found the combination of amivantamab and chemotherapy compared to chemotherapy alone showed improvement in outcome measures in previously untreated, advanced NSCLC with EGFR exon 20 insertions.
Image: PD
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.