Baricitinib is superior to placebo for treatment of alopecia areata
1. Oral baricitinib is superior to placebo regarding hair regrowth at 36 weeks.
2. Oral baricitinib led to lower severity of alopecia areata compared to placebo.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Alopecia areata is an autoimmune disorder that causes nonscarring hair loss that can affect any hair-bearing site, which can cause emotional and psychosocial distress. Extensive alopecia areata is unlikely to be remit without treatment, which currently consists of glucocorticoids, other immunosuppressive agents, and contact immunotherapy, which are not approved by the FDA. Baricitinib, a reversible JAK1, and JAK2 inhibitor, has been posited to reverse hair loss in patients with alopecia areata in previous reports. However, there is a gap in knowledge as to understanding Baricitinib’s effectiveness in causing hair regrowth among patients with alopecia areata. This study found that once-daily oral Baricitinib was reported to be superior to placebo regarding hair regrowth after 36 weeks. This study was limited by factors such as some assessments being performed remotely due to the Covid-19 pandemic, as well as a selective study population since patients with a previous inadequate response to oral JAK inhibitors and patients with an episode lasting at least 8 years without any hair regrowth were excluded from the study. Nevertheless, these study’s findings are significant, as they demonstrate that Baricitinib was superior to placebo with respect to hair regrowth in patients with alopecia areata.
Click to read the study in NEJM
Relevant Reading: Genetic Susceptibility to Alopecia
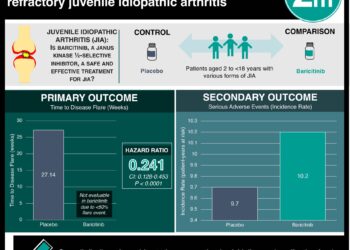
In-Depth [randomized control trial]: This randomized control trial studied 1200 patients with severe alopecia areata. Patients who had a Severity of Alopecia Tool (SALT) score of 50 or higher, with 0 representing no scalp hair loss and 100 representing complete scalp hair loss, and a current episode of alopecia areata lasting more than 6 months to less than 8 years without spontaneous improvement during the previous 6 months were eligible for the study. Patients who had a diffuse pattern of alopecia areata, or had been treated with agents such as topical glucocorticoids within 1 week before randomization, systemic glucocorticoids within 8 weeks before randomization, and JAK inhibitors within 4-8 weeks before randomization, or had an inadequate response to oral JAK inhibitors, were excluded from the study. The primary outcome measured was a SALT score of 20 or less at week 36, which has been identified as a meaningful treatment outcome for patients with severe alopecia areata. Outcomes in the primary analysis were assessed via graphical testing schemes and efficacy analysis with the use of logistic regression and analysis of covariance. Based on the analysis and two trials (BRAVE-AA1 and BRAVE-AA2) conducted, 38.8% of patients receiving daily 4mg baricitinib, 22.8% of patients receiving daily 2mg baricitinib, and 6.2% of patients receiving placebo had a SALT score of 20 or less at week 36 in the BRAVE-AA1 trial. In the BRAVE-AA2 trial, 35.9% of patients receiving 4mg baricitinib, 19.4% of patients receiving daily 2mg baricitinib, and 3.3% of patients receiving placebo had a SALT score of 20 or less at week 36. The difference between 4mg baricitinib and placebo was 32.6% (95% Confidence Interval [CI], 25.6 to 39.50 and the difference between 2mg baricitinib and placebo was 16.6% (95% CI, 9.5 to 23.8) in the BRAVE-AA1 trial. In the BRAVE-AA2 trial, the difference between 4mg baricitinib and placebo was 32.6% (25.6 to 39.6) and 16.1% (95% CI, 9.1 to 23.2) between 2mg baricitinib and placebo. Overall, this study demonstrated that once-daily oral baricitinib was superior to placebo with respect to hair regrowth, though longer trials are necessary to determine the efficacy and safety of baricitinib for alopecia areata, with trial lengths of 200 weeks recommended.
Image: PD
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.