Biosimilar drugs undergo rigorous clinical testing and yield comparable outcomes to biologics
1. Cancer biosimilar treatment trials have clinical outcomes that do not differ statistically from reference biologic products
2. The design elements of clinical trials for biosimilar drugs are rigorous
Evidence Rating Level: 1 (Excellent)
Study Rundown: Biologic treatments constitute a large portion of prescription costs in the United States. Biosimilar drugs are intended to reduce costs but are required to undergo stringent testing before approval. This systematic review and meta-analysis aimed to compare the study design of cancer biosimilar efficacy trials with reference drug trials on measures of population size, randomization and blinding. The effectiveness of biosimilars was compared with the biologic drugs as well. Reference drug trials had a lower mean number of patients than biosimilar studies (302 compared to 397, respectively). Biosimilar studies were randomized clinical trials (RCTs) more often than were reference drug trials (100% vs. 50%, respectively). As well, they were more likely to be double-blinded; 84% of biosimilar trials, compared to 17% of reference drug trials. Six disease settings were explored to determine the efficacy of biosimilar drugs compared to biologics: non-small cell lung cancer, metastatic colorectal cancer (mCRC), ERBB2-positive early breast cancer, ERBB2-positive metastatic breast cancer, follicular lymphoma, and diffuse large B-cell lymphoma (DLBCL). Outcomes showed similar efficacy between biosimilars and their biologic originators in all settings. Limitations of this study include the inability to recover complete publications for several of the trials in the sample. Additionally, because the study was comparing biologics with biosimilars, there were only 3 products available (bevacizumab, trastuzumab, and rituximab). This study only explored the efficacy of biosimilars and did not include other measures, such as toxicity or immunogenicity. As such, care should be taken when interpreting the results. Overall, the clinical outcomes did not differ statistically from the reference biologic products based on efficacy.
Click to read the study in JAMA Oncology
Click to read an accompanying editorial in JAMA Oncology
Relevant Reading: Biosimilars in oncology in the United States: a review
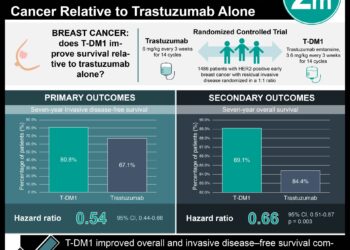
In-Depth [meta-analysis]:This systematic review and meta-analysis included 31 cancer biosimilar trials on 3 reference products with a total of 12,310 patients. Six reference drug trials with 1,811 patients were also included as a comparison. The design elements explored included population size, randomization, and blinding of the studies. Biosimilar trials had a greater average number of patients (397) compared to reference biologic trials (302). Additionally, trials for biosimilars were both more likely to be RCTs (100% vs. 50%) and double-blinded (84% vs. 17%) than reference biologic studies. The overall response rate (ORR) of bevacizumab biosimilar vs. the biologic was 1.02 (95% confidence interval (CI), 0.94-1.10) for non-small cell lung cancer (NSCLC) and 0.95 (95% CI, 0.72-1.24) for metastatic colorectal cancer (mCRC). The ORR of trastuzumab biosimilar vs. the biologic was 1.01 (95%, 0.94-1.08) for ERBB2-positive metastatic breast cancer. The ORR of rituximab biosimilar vs. the biologic was 1.04 (95% CI, 1.00-1.08) for follicular lymphoma (FL), 1.01 (95% CI, 0.96-1.05) for diffuse large B-cell lymphoma, and 0.99 (95% CI, 0.81-1.22) for chronic lymphocytic leukemia. The meta-analysis revealed the effectiveness of biosimilars was statistically comparable to the biologic drugs for all three reference products (bevacizumab, trastuzumab, and rituximab).
Image: PD
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.