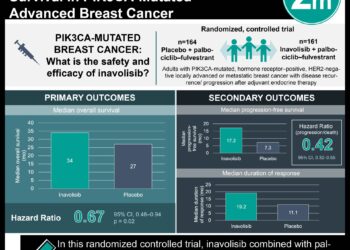
Fulvestrant for treatment of estrogen receptor-positive, HER2-negative advanced breast cancer may improve progression free survival using ESR1 mutation as guidance
1. Median progression free survival was longer in the fulvestrant and palbociclib group compared to the aromatase inhibitor plus palbociclib group.
2. Hematological toxicity was similar between groups and included neutropenia.
Evidence Rating Level: 1 (Excellent)
Study Rundown: For patients with advanced breast cancer, estrogen receptor-positive, HER2-negative tumours are the most common. As many of these patients progress on first-line treatment with aromatase inhibitors due to estrogen receptor mutations, further chemotherapy and endocrine therapies are often less effective. Mutations in the estrogen receptor α gene, ESR1, is associated with aromatase inhibitor treatment resistance and possibly more sensitive selective estrogen receptor degraders like fulvestrant. This was a two part trial: first patients were enrolled on standard treatment (CDK 4/6 inhibitor and aromatase inhibitor) and then when bESR1 levels increased with no obvious cancer progression, they were randomized in an open-label fashion to either continue with palbociclib and aromatase inhibitor or to fulvesterant and palbociclib. The main primary outcomes were progression-free survival (PFS) and the rate of grade 3 or worse adverse events (AEs). Median PFS was longer in the fulvestrant group at 11.9 months as compared to the aromatase inhibitor group at 5.7 months. Severe AEs were noted in both groups, the most common of which was neutropenia. Both groups also had instances of lymphopenia. Limitations to this study include that it was not well-powered to perform secondary endpoint analyses like overall survival and no central review for PFS. As well, the study did not assess for the estrogen receptor ESR1 mutation type, which might be less sensitive to fulvestrant. Overall, this study suggests that using a peripheral blood marker of endocrine resistance before standard measurements of progression and switching to fulvestrant prior to tumour progression can lead to longer PFS.
Click to read the study in The Lancet Oncology
Relevant Reading: Systemic therapy for estrogen receptor-positive, HER2-negative breast cancer
In-Depth [randomized controlled trial]: This randomized, phase 3 trial was conducted through 83 hospitals located in France. There were two steps involved in recruiting the patients; and 1017 adult female patients with advanced estrogen-positive, HER2-receptor negative breast who were systemic treatment-naïve or who hadn’t received adjuvant aromatase inhibitors in the previous 12 months were included and treated using aromatase inhibitor and palbociclib. The second step included 172 of those patients whose bESR1 increased during treatment in step 1; 84 were randomly assigned to receive aromatase inhibitor and palbociclib and 88 received fulvestrant and palbociclib. An optional crossover step was included for patients whose disease progressed on aromatase inhibitor and palbociclib treatment, the outcome of which would describe the benefit of switching therapies after an increase in bESR1. Median PFS in the fulvestrant group was 11.9 months (95% confidence interval (CI), 9.1-13.6 months) as compared to 5.7 months in the aromatase inhibitor group (95% CI, 3.9-7.5 months) (stratified hazard ratio (HR), 0.61; 95% CI, 0.43-0.86). Fewer patients in the fulvestrant group had PFS events as compared to the aromatase inhibitor group (72.7% vs. 83.3%, respectively). 41.7% of patients in the aromatase inhibitor and palbociclib group, while 44.3% of the fulvestrant and palbociclib group developed neutropenia. Lymphopenia was noted in 3.6% of the aromatase inhibitor group and in 4.5% of the fulvestrant group. During the study, 45 patients in the second step of the study died, primarily due to cancer; 21 in the fulvestrant group and 24 in the aromatase inhibitor group. 68% of the 69 patients whose diseased progressed received cross-over treatment with fulvestrant and palbociclib. The median second PFS in this group was 3.5 months (95% CI, 2.7-5.1 months).
Image: PD
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.