Optimizing Subtyping-Based Therapy in Triple-Negative Breast Cancer
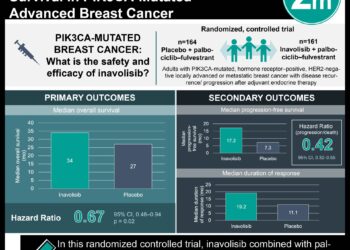
1. The median progression-free survival was improved with the pooled subtyping-based therapy group compared to the control group (11.3 months vs 5.8 months with an HR 0.44).
2. Grade 3-4 adverse events occurred in 57% of the subtyping-based group and 31% in the control group with the most common being neutropenia, anemia, and increased alanine aminotransferase.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Triple-negative breast cancer is a challenging and aggressive subtype, primarily treated with chemotherapy or more recently immune checkpoint inhibitors, however, many lack the relevant biomarker, PD-L1. Recent studies identified four subtypes; luminal androgen receptor (LAR), immunomodulatory, basal-like immune-suppressed (BLIS), and mesenchymal-like (MES). This study aimed to evaluate subtyping-based therapy’s efficacy and safety as the first-line treatment for metastatic triple-negative breast cancer compared to a taxane. The primary endpoint was progression-free survival (PFS), and secondary outcomes included overall survival (OS), objective response rate (ORR), disease control rate (DCR), duration of response (DoR), and safety. PFS was 11.3 months in the pooled subtyping-based group vs 5.8 months in the control group, with an HR 0.44 (p<0.0001). This improvement in PFS was consistent across all subgroup analyses. ORR was 80% in the pooled subtyping-based group vs 44.8% in the control group, with a DCR of 91.7% vs 48.3%, respectively. Median DoR was 12.0 months in the pooled subtyping-based group vs 4.2 months, with an HR 0.44. Median OS was not reached in either group with HR 0.82 for the current analysis. With regards to safety, 57% in the subtyping-based group and 31% in the control group had grade 3–4 events, with the most common being neutropenia (30% vs 23%), anemia (7% vs 0%), and increased alanine aminotransferase (6% vs 1%). Additionally, a post-hoc analysis suggested the presence of baseline NF1, PIK3R1, ATR, and POLE somatic mutations was negatively associated with prognosis. The strengths of this study included its multi-cohort methodology, and the limitations included a small sample size especially in certain small subgroups, and the study did not compare to the standard of care in North America which is the use of pembrolizumab in PD-L1 positive patients. Overall, this study found outcome improvements with subtyping-based precision treatment compared with standard chemotherapy in the first-line treatment of metastatic triple-negative breast cancer.
Click to read the study in Lancet
Click to read an accompanying editorial in Lancet
Relevant Reading: Genomic and Transcriptomic Landscape of Triple-Negative Breast Cancers: Subtypes and Treatment Strategies
In-Depth [randomized controlled trial]: This open-label, phase 2 trial enrolled adults with histologically confirmed metastatic or recurrent triple-negative breast cancer who had a 6-month washout period from their previous treatment and using next-generation sequencing, enrolled each patient into 5 cohorts (LAR- HER2 mut [3 patients], LAR- PI3K/AKT mut [18 patients], immunomodulatory [59 patients], BLIS/MES- PI3K/AKT WT [58 patients], and MES- PI3K/ AKT mut [1 patient]) and then further randomized (1:1) them into either receiving nab-paclitaxel (control group) or nab-paclitaxel plus a subtyping-based regimen: pyrotinib (LAR- HER2 mut), everolimus (LAR- PI3K/AKT mut), camrelizumab and famitinib (immunomodulatory), bevacizumab (BLIS/MES- PI3K/AKT WT), and everolimus (MES- PI3K/AKT mut). The median follow-up time was 22.5 months. PFS was 11.3 months (95%CI, 8.6-15.2) in the pooled subtyping-based group vs 5.8 months (4.0-6.7) in the control group, with an HR 0.44 (95%CI, 0.30-0.65, p<0.0001). This improvement in PFS was consistent across all subgroup analyses (except for lack of assessment due to the small sample size in LAR- HER2 mut and MES- PI3K/AKT mut). ORR was 80% (95%CI, 67.7-89.2) in the pooled subtyping-based group vs 44.8% (31.7-58.5) in the control group, with an OR 4.92 (95%CI, 2.17-11.15). DCR was 91.7% (95%CI, 86.1-97.2) vs 48.3% (34.5-61.8), respectively. Median DoR was 12.0 months (95%CI, 7.2-16.5) in the pooled subtyping-based group vs 4.2 months (2.2-5.9), with an HR 0.44 (95%CI, 0.26-0.78). Median OS was not reached in either group with HR 0.82 (95%CI, 0.38-1.56) for the current analysis. With regards to safety, 57% in the subtyping-based group and 31% in the control group had grade 3–4 events, with the most common being neutropenia (30% vs 23%), anemia (7% vs 0%), and increased alanine aminotransferase (6% vs 1%). Additionally, a post-hoc analysis suggested the presence of baseline NF1, PIK3R1, ATR, and POLE somatic mutations was negatively associated with prognosis. Overall, this study found outcome improvements with subtyping-based precision treatment compared with standard chemotherapy in the first-line treatment of metastatic triple-negative breast cancer.
Image: PD
©2024 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.




![Galangin may sensitize apoptosis-resistant renal carcinoma cells [PreClinical]](https://www.2minutemedicine.com/wp-content/uploads/2016/01/Papillary_renal_cell_carcinoma_-_very_high_mag-75x75.jpg)

