Second-line tisagenlecleucel does not improve outcomes in B-cell lymphoma
1. Patients with B-cell non-Hodgkin’s lymphoma receiving tisagenlecleucel did not show delayed lymphoma progression compared to those receiving standard salvage therapy.
2. Tisagenlecleucel treatment did not show improved event-free survival in patients with B-cell non-Hodgkin’s lymphoma compared to standard therapy.
Evidence Rating Level: 1 (Excellent)
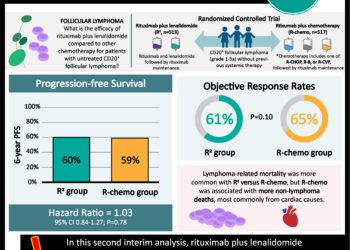
Study Rundown: Tisagenlecleucel is an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy that has recently been approved for third-line use in patients with diffuse B-cell lymphoma; however, its efficacy as a second-line therapy is unknown. To investigate this, the BELINDA randomized controlled trial was conducted and randomized patients with B-cell non-Hodgkin’s lymphoma who failed standard first-line therapy, in a 1:1 ratio, to receive either tisagenlecleucel or standard salvage chemotherapy including the option for autologous hematopoietic stem-cell transplantation (HSCT). The primary endpoint for this study was event-free survival after 12 weeks, while secondary endpoints analyzed the response to treatment and safety. The study found that the median time to event-free survival did not significantly differ between groups, in fact, a greater proportion of patients receiving tisagenlecleucel experienced lymphoma progression at week 6. No differences in response rate were appreciated between groups and severe side-effect profiles were also similar. Altogether, the study fails to generate support for use of tisagenlecleucel as second-line therapy for patients with diffuse B-cell lymphoma; however, the authors suggest that a smaller subset of patients may benefit. This study was limited by a lack of proper stratification for other tumor biology characteristics, which may have influenced worse outcomes in the experimental group.
Click to read the study in NEJM
Relevant Reading: Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma
In-Depth [ randomized controlled trial]: In this multi-center, randomized control trial named the JULIET trial, 322 patients with histologically confirmed aggressive B-cell lymphoma that did not respond to the conventional first-line treatment were randomized in a 1:1 ratio to either receive tisagenlecleucel, an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy or standard rescue chemotherapy with the option for autologous hematopoietic stem-cell transplantation (HSCT). Groups were stratified according to geographic region, time since last first-line treatment, and International Prognostic Index (IPI) score. For the primary outcome, an independent review committee determined disease progression based upon positron-emission tomography-computed tomography to determine event-free survival at 12 weeks follow-up. Secondary endpoints assessed the percentage of patients with a response to therapy, and overall safety profile as indicated by severe side effects. Statistical analyses utilized the stratified log-rank test to assess differences in event-based survival and stratified Cox regression to estimate hazard ratios. The study found that second-line tisagenlecleucel was unable to demonstrate differences in event-free survival from standard rescue chemotherapy as both groups had a median event-free survival of 3 months (P=0.61). The stratified adjusted hazard ratio for tsiagenlecleucel was 0.99 (95% confidence interval [CI], 0.83 – 1.85). In fact, at week 6, 38.3% of patients responded to tisagenleuleucel compared to 53.8% in the standard care group with this control group experiencing a lower proportion of progressive disease at this timepoint. Almost all patients in the study (98.8%) experienced an adverse side effect, and 84% of patients in the tisagenlecleucel group experienced a severe adverse event (grade 3 or higher) attributed to treatment. Taken together, this study fails to support the use of tisagenlecleucel CAR-T therapy as a second-line option for patients with aggressive B-cell lymphoma given its lack of efficacy and high adverse event rate. The study proposes that while this group as a whole may not benefit, further analyses may identify subsets of patients that may benefit from this treatment regimen.
Image: PD
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.