2 Minute Medicine Rewind May 4 – May 11, 2014
Image: PD
In this section, we highlight the key high-impact studies, updates, and analyses published in medicine during the past week.
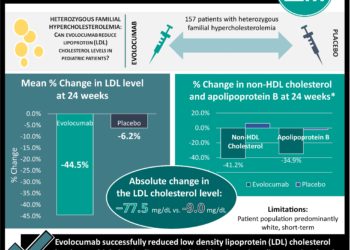
A 52-Week Placebo-Controlled Trial of Evolocumab in Hyperlipidemia
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is an integral protein in the LDL cholesterol degradation pathway, and its degradation has been postulated to decrease LDL cholesterol. In this multicenter randomized trial, 901 patients were randomized to evolocumab (antibody to PCSK9) verses placebo after initial run-in period of 4 to 12 weeks of atorvastatin 10mg, atorvastatin 80mg, atorvastatin 90mg and ezetimibe 10mg, or diet alone. The overall reduction from baseline LDL cholesterol in the evolocumab group was 57.0% (p < 0.001), and there was significant decreases from baseline LDL cholesterol regardless of the initial run-in therapy (p < 0.001). Over the course of a year, there were only minor adverse events including upper respiratory tract infections, influenza, and back pain. PCSK9 is an promising target for LDL cholesterol reduction, and in addition to this monoclonal antibody from Amgen, Sanofi (SAR236553), Pfizer (RN316), Roche (RG7652), and Merck (1D05-IgG2) have therapeutics in the development pipeline.
Changes in Mortality After Massachusetts Health Care
The 2006 Massachusetts health care reform law with increased access to care is an early model of the Affordable Care Act. In this retrospective cohort analysis of adults in Massachusetts counties, two cohorts (before reform [2001 to 2005] and after reform [2007 to 2010]) were compared with control counties in other states. Reform in Massachusetts was associated with a decrease in all-cause mortality (-2.9%, p = 0.003) or an absolute decrease of 8.2 deaths per 100,000 adults. Changes were larger in counties with lower household incomes and higher uninsured rates. The number needed to treat was 830 adults gaining health insurance to prevent 1 death per year. Health care reform in Massachusetts was associated with reductions in all-cause mortality.
Transcatheter Aortic-Valve Replacement with a Self-Expanding Prosthesis
Transcatheter aortic-valve replacement (TAVR) has been suggested as an alternative to surgical aortic valve replacement in high risk patients with severe aortic stenosis, however while many studies have suggested equivalence in patient outcomes, it is still controversial whether one is superior. In this multicenter randomized trial of 795 patients in the US, patients with severe aortic stensosi and deemed higher risk were randomized to either TAVR with self-expanding bioprosthesis or surgical aortic valve replacement. At one year, the mortality was significantly lower in the TAVR group (14.2% v. 19.1%, p = 0.04 for superiority). There was not an increase in the risk of stroke, and echographic indexes were equivalent. Further developments in TAVR technology and investigation of the relative merits of surgical vs. transcatheter replacement is warranted, particularly in high risk patients with severe aortic stenosis.
Effect of Fluconazole Prophylaxis on Candidiasis and Mortality in Premature Infants
Invasive candidiasis is a cause of death and neurologic impairment in preterm and low weight premature infants. In the multicenter, blinded, randomized trial, 361 premature infants weighing less than 750g at birth were randomized to receive fluconazole or placebo twice weekly for 6 weeks. At 49 days, the primary endpoint of death or invasive candidiasis was not statistically significant (16% vs. 21% in placebo, p = 0.24). There was less invasive candidiasis (3% vs. 9%, p = 0.02), however neurodevelopmental impairment was not improved with fluconazole (31% vs. 27%, p = 0.60). There does not appear to be strong evidence for prophylacitc fluconazole in all low birth weight infants.
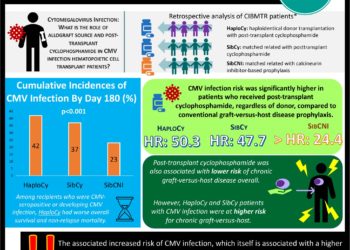
Letermovir for Cytomegalovirus Prophylaxis in Hematopoietic-Cell Transplantation
Cytomegalovirus (CMV) infection is a significant concern in immunocompromised hosts, including patients undergoing hematopoietic-cell transplantation. Letermovir (AIC246) is a novel drug from the prophylaxis of CMV. In this randomized trial of 131 seropositive recipients of hematopoetic-cell transplantation, patients received varying daily oral doses of letermovir (60, 120, 240mg) or placebo for 12 weeks after engraftment. There was a dose response curve to all-cause prophylaxis failure (CMV infection) with 64% for placebo, 48% at a dose of 60mg (p = 0.32), 32% at a dose of 120mg (p = 0.01), and 29% at a dose of 240mg (p = 0.007). The number and types of adverse events were similar in the placebo and letermovir arms. Further study should be undertaken to understand whether all patients undergoing hematopoietic-cell transplantation should all have CMV prophylaxis with letermovir.
©2012-2014 2minutemedicine.com. All rights reserved. No works may be reproduced without written consent from 2minutemedicine.com. Disclaimer: We present factual information directly from peer reviewed medical journals. No post should be construed as medical advice and is not intended as such by the authors or by 2minutemedicine.com. PLEASE SEE A HEALTHCARE PROVIDER IN YOUR AREA IF YOU SEEK MEDICAL ADVICE OF ANY SORT. Content is produced in accordance with fair use copyrights solely and strictly for the purpose of teaching, news and criticism. No benefit, monetary or otherwise, is realized by any participants or the owner of this domain.