Cardiovascular outcomes of dialysis patients using phosphate binders with or without calcium
1. In this observational cohort study of patients with end-stage renal disease (ESRD) undergoing hemodialysis, there was no observed difference in cardiovascular events for patients receiving calcium-containing or calcium-free phosphate binders.
2. There was no difference in outcomes observed in pre-specified subgroup analysis based on sex or age group.
Evidence Rating Level: 2 (Good)
Study Rundown: High serum phosphate is a common complication of ESRD and is associated with increased cardiovascular mortality. Traditional methods to reduced phosphate levels include dietary changes and oral phosphate binders. The current Kidney Disease: Improving Global Outcomes (KDIGO) clinical guidelines recommend against calcium containing phosphate binders due to concern for calcium related cardiovascular disease. Non-calcium containing phosphate binders are costly, and evidence for reduction in cardiovascular outcomes compared to calcium containing agents is limited. This observational cohort study of Medicare patients with ESRD initiating dialysis who were on calcium acetate or the non-calcium binder sevelamer. The study found no difference in rates of cardiovascular events between the calcium and non-calcium groups, and no difference in overall survival.
The main strengths of the study included a broadly representative US population. The limitations of the study include the non-randomized design with potential for bias, and lack of data on PTH levels and use of iron-based phosphate binders, or over-the-counter calcium carbonate.
Click to read the study in JAMA Internal Medicine
Relevant Reading: Chronic Kidney Disease Sevelamer Versus Calcium-Based Binders for Treatment of Hyperphosphatemia in CKD: A Meta-Analysis of Randomized Controlled Trials
In-Depth [retrospective cohort]: This retrospective cohort study used data from United States Renal Data System (USRDS) and linked Medicare claims from May 1, 2012 – December 31, 2013. The study population included patients 65 years or older treated with sevelamer or calcium acetate within 180 days of initiating hemodialysis. Patients were excluded if they did not have ongoing dialysis, received transplant, had a history of alcohol use, previously used phosphate binders, or did not have phosphate/calcium levels measured during the study period. The primary outcome was hazard ratio for non-fatal cardiovascular events, while all-cause mortality was the secondary outcome.
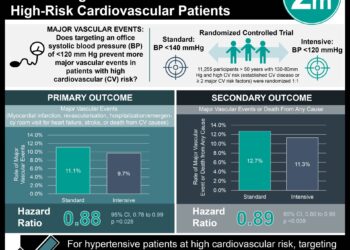
The trial included 2639 patients receiving sevelamer, and 2065 receiving calcium acetate. The incidence rate of cardiovascular events was 458 per 1000 person-years for sevelamer and 464 per 1000 person-years for calcium acetate (HR 0.96; 95%CI, 0.84-1.10). There was no observed difference in overall mortality, or in analysis divided by age group, or sex.
Image: PD
©2019 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.