Oxaliplatin-Based Adjuvant Chemotherapy for Rectal Cancer After Preoperative Chemoradiotherapy (ADORE): Long-Term Results of a Randomized Controlled Trial
1. Adjuvant FOLFOX improves disease-free survival in patients with pathologic Stage II or III rectal cancer after upfront surgery and preoperative chemoradiotherapy, but is associated with a higher rate of adverse events.
Evidence Rating Level: 1 (Excellent)
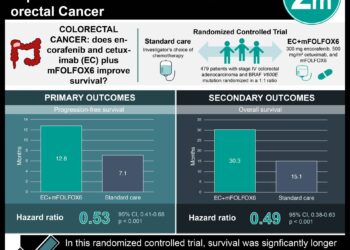
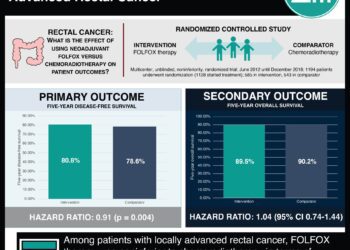
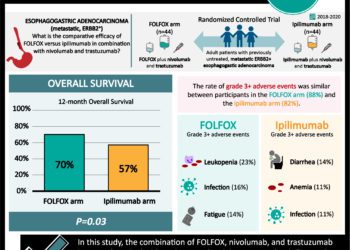
Postoperative pathologic stage is one of the most important prognostic factors in patients with rectal cancer after surgery and preoperative chemoradiotherapy (CRT). Adjuvant chemotherapy is also recommended after surgery for patients with postoperative pathologic stages II and III rectal cancer. And, although only adjuvant fluoropyrimidines have demonstrated a survival benefit, oxaliplatin-based regimens are widely used in this setting based on the extrapolation of results from patients with colon cancer. In addition, most previous studies of oxaliplatin-based regimens have been designed based on clinical rather than pathologic staging. In this multicenter, randomized, controlled trial, 321 patients with postoperative ypStage II (ypT3-4N0) or III (ypTanyN1-2) rectal cancer after fluoropyrimidine-based preoperative CRT and total mesorectal excision (TME) were randomized to receive adjuvant chemotherapy with either fluorouracil and leucovorin (FL) or FOLFOX (oxaliplatin, leucovorin, and fluorouracil) to study disease-free survival (DFS). Researchers found that at 6 years of follow-up, the DFS rate was higher in the FOLFOX group than in the FL group (68.2% vs. 56.8%, respectively, HR 0.63, 95% CI 0.43 to 0.93, p=0.018). There was no statistically significant difference in 6-year overall survival (OS) between the two groups (FOLFOX 78.1% vs. FL 76.4%, HR 0.73, 95% CI 0.45 to 1.19, p=0.21). Exploratory subgroup analysis of DFS also revealed that FOLFOX treatment was favorable compared with FL in patients with ypStage III disease, male gender, age younger than 65 years, advanced pathologic stages, high-grade histology, minimally regressed tumor, an absence of lymphovascular or perineural invasion, and a longer than 6-week interval between CRT and surgery. Neutropenia (69.9% vs. 45.6%, p<0.001) and thrombocytopenia (26.0% vs. 2.0%, p<0.001) were, however, more common in the FOLFOX group, as were fatigue (28.3% vs. 17.4%, p=0.037), nausea (53.4% vs. 37.6%, p=0.007), and sensory neuropathy (70.5% vs. 5.4%, p<0.001); however, there was no significant difference in the occurrence of grade 3 or 4 adverse events between the two groups. Overall, this study suggests that adjuvant FOLFOX may improve disease-free survival in patients with postoperative ypStage II or III rectal cancer who received preoperative CRT and TME, but is associated with a higher rate of adverse events.
Click to read the study in the Journal of Clinical Oncology
Image: PD
©2019 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.