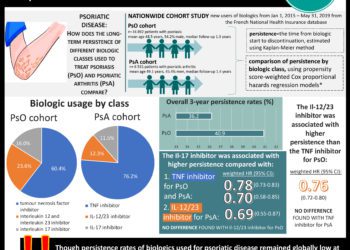
Tag: IL-17 inhibitor
Sonelokimab 120 mg or less showed significant clinical benefit over placebo in patients with plaque psoriasis
1. At 12 weeks, the proportion of patients with an IGA score of 0 or 1 was significantly greater in ...
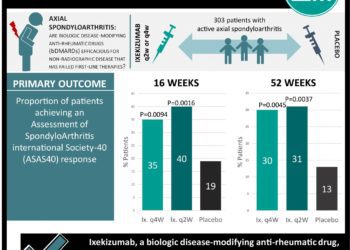
#VisualAbstract: Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X)
1. Ixekizumab, an IL-17 inhibitor, elicits disease response in patients with non-radiographic active axial spondyloarthritis who have failed treatment with ...
Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X)
1. Ixekizumab, an IL-17 inhibitor, elicits disease response in patients with non-radiographic active axial spondyloarthritis who have failed treatment with ...
2 Minute Medicine Rewind December 9, 2019
Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial 1. Obeticholic ...