#VisualAbstract: Bimekizumab more efficacious than ustekinumab in treating severe plaque psoriasis
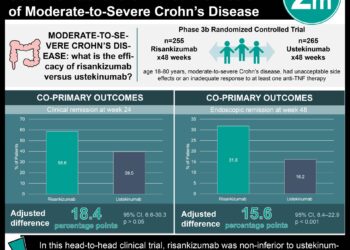
1. Bimekizumab showed higher efficacy in the treatment of severe plaque psoriasis than ustekinumab with patients showing greater proportion of PASI90 and IGA response.
2. The safety profile of Bimekizumab was overall mild and similar to current treatment option ustekinumab
Evidence Rating Level: 1 (Excellent)
Study Rundown: Plaque psoriasis is a chronic inflammatory skin disease associated with a number of other serious conditions, including cardiac disease, inflammatory bowel disease and malignancies. Although there are several treatment options with biologics currently available, certain populations of patients have shown particularly poor responses to these treatments, leading to a search for new alternatives. Bimekizumab is a monoclonal IgG1 selective antibody that has shown promise in preclinical trials in reducing pro-inflammatory cytokines and psoriasis-linked genes. This phase 3 randomized, double-blind, active comparator and placebo-controlled trial compared the use of bimekizumab versus placebo and versus actor coparator ustekinumab, a common treatment for psoriasis, in patients with moderate to severe disease. Overall, bimekizumab showed improved efficacy in achieving complete skin clearance versus placebo and active comparator. Responses achieved with Bimekizumab were attained more quickly and maintained for a longer period than with placebo or ustekinumab. Likewise, patients who switched from placebo to Bimekizumab after a 16-week period showed rapid improvement in their response to treatment. The safety profile across all three groups was similar; most common treatment-related events included nasopharyngitis, oral candidiasis and upper respiratory infections. A total of five patients receiving Bimekizumab and four receiving ustekinumab experienced serious adverse events. Two important limitations of this study include the relatively short length of time (52 weeks) as well as the strict inclusion criteria, potentially limiting study generalizability.
Click to read the study in The Lancet
In-Depth [randomized controlled trial]: This phase 3, multicenter, randomized, placebo and active comparator-controlled trial took place in 105 sites and recruited a total 567 participants with moderate to severe plaque psoriasis. Participants were randomized to receive bimekizumab, ustekinumab, or placebo in a 4:2:1 ratio. Those in the placebo group would switch to taking Bimekizumab 16 weeks after trial start. Coprimary endpoints were proportions of patients with >90% improvement from baseline PASI score (PASI90), and proportion of patients with an IGA response improved by at least two category levels, at 16 weeks.
Overall, patients in the bimekizumab group had a greater proportion of patients achieving PASI90 (Bimekizumab vs ustekinumab risk difference = 35 [95% CI 27-43], p<0.0001 and Bimekizumab vs placebo risk difference=80 [95% CI 74-86], p<0.0001) and IGA responses (Bimekizumab vs ustekinumab risk difference=30 [95% CI 22-39], p<0.0001 and Bimekizumab vs placebo risk difference=79 [95% CI 73-85], p<0.0001) at week 16. Significantly more patients in the bimekizumab group had complete skin clearance (PASI100) at week 16 versus ustekinumab (nominal p<0.0001) and placebo (nominal p<0.0001). At week 52, a higher proportion of patients in the Bimekizumab group had maintained responses, for both PASI90 (p<0.0001) and IGA response (p<0.0001) compared to those taking ustekinumab. Overall, the safety profile of each group was similar. Discontinuations due to treatment-related adverse events were higher in the placebo group than the Bimekizumab and ustekinumab groups. In the first 16 weeks, 56% of patients in the Bimekizumab group, 51% of patients in the ustekinumab group and 47% of patients in the placebo group reported experiencing treatment-related adverse events; serious treatment-related adverse events, however, were only reported in 2%, 3% and 2% of patients in each group respectively.
©2020 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.