Adavosertib compared with active monitoring is potentially effective against TP53/RAS-mutant metastatic colorectal cancer.
1. Patients on adavosertib had both increased progression-free survival and better tumor control, as compared to patients being actively monitored only.
2. Overall survival was not affected by adavosertib as compared to active monitoring, but a subgroup analysis showed an increase in survival for patients with a left-sided primary compared to those with a right-sided primary.
Evidence Rating Level: 2 (Good)
Study Rundown: Metastatic colorectal cancer patients with the TP53/RAS-mutant are considered a worse prognosis group as compared to either mutation subgroup alone. This study hypothesized that the TP53/RAS mutation would be sensitive to a WEE1 kinase inhibitor, adavosertib (AZD1775). Comparison of progression-free survival (PFS), overall survival (OS), and tumor control between a treatment arm (adavosertib) and a control arm (active monitoring) showed that both PFS and tumor control were increased with administration of adavosertib in both intention-to-treat (ITT) and per-protocol-analysis (PPA). Overall survival was similar between study groups. Secondary outcomes of toxicity were increased in the treatment group as compared to active monitoring. Adverse events included nausea, vomiting, diarrhea, and fatigue. A major limitation of this study is that it was unblinded in order to provide supportive care for those on adavosertib. Other studies have shown that antiemetics were required to control the severe nausea and vomiting associated with this treatment. Further limitations include the lack of generalizability of results to the entire TP53/RAS-mutant group because the eligibility requirements of this study necessitated stability following induction chemotherapy, thereby excluding patients of this subgroup with the worst prognosis. In general, however, adavosertib provides increased progression-free survival and tumor control in patients with TP53/RAS-mutant mCRC.
Click to read the study in JCO
Relevant Reading: MK-1775, a Potent Wee1 Inhibitor, Synergizes with Gemcitabine to Achieve Tumor Regressions, Selectively in p53-Deficient Pancreatic Cancer Xenografts
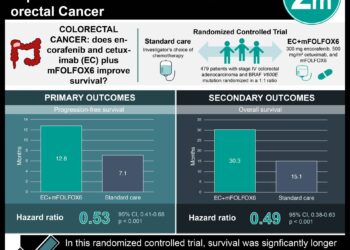
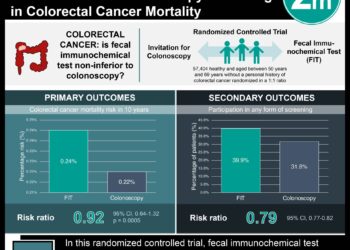
In-Depth [randomized controlled trial]: This phase 2, open-label, randomized trial of 69 patients with TP53/RAS-mutant mCRC comparing adavosertib treatment to active monitoring (AM) on outcomes of progression-free survival (PFS), overall survival (OS), and tumor control. A 2:1 ratio was used to randomize patients to the study arm compared to AM. Eligibility for inclusion in this FOCUS4-C study included adult patients with both the TP53 and RAS somatic mutations of mCRC who were stable at 16 weeks post-chemotherapy induction, as assessed by CT. Patients in the treatment arm received antiemetic therapy including oral dexamethasone as well as oral 5HT3 antagonist. Patients remained on treatment until disease progression or severe toxicity. Intention-to-treat analysis of PFS was consistent with the PPA (unadjusted HR = 0.55; 95% CI, 0.32 to 0.94; p=0.032; adjusted HR = 0.40; 95% CI, 0.21 to 0.75; p=0.0051). Tumor control was increased in the treatment arm. No overall survival benefit was noted (unadjusted HR = 0.79; 95% CI, 0.42 to 1.48, p=0.47; adjusted HR = 0.92; 95% CI, 0.44 to 1.94, p=0.93), however, a subgroup analysis comparing OS based on the sidedness and compared to active monitoring showed an increase in OS for patients with left-sided tumors (adjusted HR = 0.37; 95% CI, 0.15 to 0.87) and a decreased OS for patients with right-sided tumors (HR = 6.5; 95% CI, 0.72 to 6.43) with caution about the interpretation given the low number of events. The level of reported toxicity, while higher in the treatment arm, was mostly low grade. Between 2-10% of the patients in the treatment arm had grade 3 or higher, while none of the AM group did.
Image: PD
©2021 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.