Adjuvant hormone therapy may improve survival in ovarian cancer
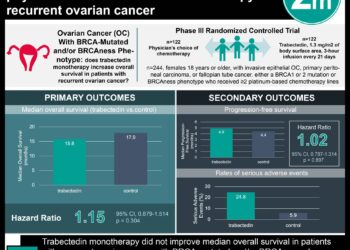
1. In a phase III trial of 150 patients with epithelial ovarian cancer, the use of adjuvant hormone therapy was associated with an increase in overall survival compared to the control group.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Ovarian cancer remains as the most common cause of gynecological cancer-related mortality among women worldwide. Definitive surgical ovarian cancer treatment in pre-menopausal women results in premature menopause and an increased risk for vasomotor symptoms, coronary heart disease, and osteoporosis. While hormone replacement therapy (HRT) can reduce these adverse effects, there is uncertainty whether HRT may increase the risk of ovarian cancer relapse. The purpose of the Adjuvant Hormone Therapy (AHT) trial was to evaluate the impact of HRT on survival in patients with ovarian cancer.
This trial randomized 150 ovarian cancer patients to receive either HRT or placebo. At the conclusion of the trial, the patients in the HRT group demonstrated significant increased overall and relapse-free survival compared to the control group. The effect remained after adjusting for known prognostic factors such as disease stage and residual tumor bulk. The results of this study support the use of HRT for patients who demonstrate severe menopausal symptoms after ovarian cancer treatment. This is the largest randomized trial assessing the use of hormone therapy among women with ovarian cancer, and has broad implications for the long-term management of these patients and treatment options to improve their quality of life. However, the study is limited by the relatively small sample size, which did not meet its original recruitment goals. Additional large randomized trials may be required to confirm this association.
Click to read the study in JCO
Relevant Reading: Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative
In-Depth [randomized controlled trial]: The Adjuvant Hormone Therapy (AHT) trial was a phase III, multi-center, randomized controlled trial that compared the use of HRT versus control in patients with epithelial ovarian cancer. The study recruited a total of 150 patients. Of these, 34 were premenopausal and 116 were postmenopausal. The primary end point was overall survival (OS) from randomization to death of any cause. Secondary end points included relapse-free survival (RFS), compliance to hormone treatment, and frequency of adverse events (myocardial infarction, fracture, transient ischemic attack, cerebrovascular accident, second cancer). At a median follow up of 19.1 years with intention to treat analysis, OS was superior in the HRT group (HR: 0.63; 95% CI: 0.44-0.9, p=0.011) compared to control. Interestingly, RFS was superior in the HRT group (HR: 0.67; 95% CI: 0.47-0.97, p = 0.032). Adverse event rates were low in both the HRT (12%) and control group (16%) with no significant difference between study arms (p=0.64).
Image: PD
©2015 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.