Amivantamab Plus Chemotherapy with and without Lazertinib in EGFR-mutant NSCLC
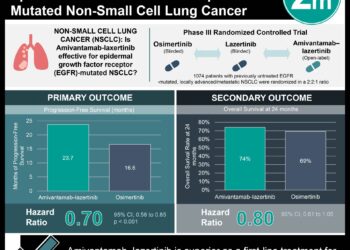
1. Progression-free survival was 6.3 months for the amivantamab–chemotherapy group, 8.3 months for the amivantamab–lazertinib–chemotherapy group, and 4.2 months for the chemotherapy group.
2. Adverse events grade 3 or higher occurred in 72% vs 92% vs 48% respectively, with the most common adverse events being neutropenia, thrombocytopenia, anemia, and leukopenia
Evidence Rating Level: 1 (Excellent)
Study Rundown: Osimertinib is the standard first-line treatment for EGFR-mutated non-small-cell lung cancer (NSCLC), but resistance often develops. Amivantamab, an EGFR-MET bispecific antibody, and lazertinib, another third-generation tyrosine kinase inhibitor (TKI), have shown efficacy individually. This study assessed amivantamab–chemotherapy (AC) vs amivantamab–lazertinib–chemotherapy (ALC) vs chemotherapy alone (C) in patients with EGFR-mutated advanced NSCLC who have progressed after osimertinib monotherapy. The primary endpoint was progression-free survival (PFS) and secondary endpoints included objective response rate (ORR), duration of response (DoR), overall survival (OS), and safety. The median PFS was 6.3 months for the AC group, 8.3 months for the ALC group, and 4.2 months for the C group. When comparing PFS for the AC vs C group, HR was 0.48 (p< 0.001), and when comparing ALC vs C, HR was 0.44 (p< 0.001). PFS benefit was consistent across all subgroups including brain metastases, osmiertinib line of therapy, and EGFR mutation. Median intracranial PFS was 12.5 months for AC, 12.8 months for ALC, and 8.3 months for C. Adverse events grade 3 or higher occurred in 72% of patients with AC, 92% with ALC, and 48% with C, with most common grade 3 or higher adverse events being neutropenia (45% vs 55% vs 21% respectively), thrombocytopenia (15% vs 37% vs 9%), anemia (12% vs 18% vs 9%), and leukopenia (20% vs 27% vs 9%). Strengths of this study included its methodology and sample size, and limitations of this study included its immature data. Overall, it was found that combination therapy, either amivantamab–chemotherapy or amivantamab–lazertinib–chemotherapy, had improved outcomes when compared to chemotherapy alone in patients with locally advanced or metastatic EGFR-mutated NSCLC with disease progression after standard osimertinib monotherapy.
Click to read the study in Annals of Oncology
Relevant Reading: Combining osimertinib with chemotherapy in EGFR-mutant NSCLC at progression
In-Depth [randomized controlled trial]: This phase III trial enrolled adults with locally advanced or metastatic EGFR-mutated (Ex19del or L858R) NSCLC with disease progression after osimertinib monotherapy and randomized them into three groups in a 2:2:1 ratio; ALC (263 patients), C (263), or AC (131). Chemotherapy included pemetrexed and carboplatin. Patients with brain metastases were included. The median follow-up time was 8.7 months. Median PFS was 6.3 months (95%CI 5.6-8.4) for the AC group, 8.3 months (95%CI 6.8-9.1) for the ALC group, and 4.2 months (95%CI 4.0-4.4) for the C group. When comparing PFS for the AC vs C group, HR was 0.48 (95%CI, 0.36-0.64, p< 0.001), and when comparing ALC vs C, HR was 0.44 (95%CI, 0.35-0.56, p< 0.001). PFS benefit was consistent across all subgroups including brain metastases, osmiertinib line of therapy, and EGFR mutation. Median intracranial PFS was 12.5 months (95%CI, 10.8-NA) for AC, 12.8 months (95%CI, 11.1-14.3) for ALC, and 8.3 months (95%CI, 7.3-11.3) for C. ORR was 64% (95%CI, 55-72) for the AC group, 63% (95%CI, 57-69) for the ALC group, and 36% (95%CI, 30-42) for the C group, with OR 3.10 (95%CI, 2.00-4.80, p< 0.001) when comparing AC vs C, and OR 2.97 (95%CI, 2.08-4.24, p< 0.001) when comparing ALC vs C. Median DoR was 6.9 months (95%CI, 5.5-NA) for AC, 9.4 months (95%CI, 6.9-NA) for ALC, and 5.6 months (95%CI, 4.2-9.6) for C. Interim OS analysis showed HR was 0.77 (95%CI, 0.49-1.21) for AC vs C and 0.96 (95%CI, 0.67-1.35) for ALC vs C. Adverse events grade 3 or higher occurred in 72% of patients with AC, 92% with ALC, and 48% with C, with most common grade 3 or higher adverse events being neutropenia (45% vs 55% vs 21% respectively), thrombocytopenia (15% vs 37% vs 9%), anemia (12% vs 18% vs 9%), and leukopenia (20% vs 27% vs 9%). Overall, it was found that combination therapy, either amivantamab–chemotherapy or amivantamab–lazertinib–chemotherapy, had improved outcomes when compared to chemotherapy alone in patients with locally advanced or metastatic EGFR-mutated NSCLC with disease progression after osimertinib monotherapy.
Image: PD
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.