Antidepressant use in pregnancy not linked with autism-spectrum disorder in exposed children
1. No significant association between serotonergic antidepressant use and autism spectrum disorder risk was found between exposed and unexposed children.
Evidence Rating Level: 2 (Good)
Study Rundown: Depression is a common complication during pregnancy. While selective serotonin reuptake inhibitors (SSRIs) are used to treat depression during pregnancy, much remains unknown about any impacts on the exposed fetus. Recently, some studies have suggested a direct correlation between the maternal use of SSRIs during pregnancy and the development of autism-spectrum disorder (ASD) in children. However, many of these studies were underpowered or failed to control for potential confounders. Therefore, the current study utilizes a retrospective cohort and a variety of methods to control for confounders in order to assess the risk of ASD in children exposed to SSRIs in utero.
The study found that among pregnancies exposed to SSRIs, 2% of children were subsequently diagnosed with ASD. However, when using inverse probability weighting to control for confounders, the difference in ASD risk among exposed children was not statistically significant. Women included in the retrospective cohort tended to have lower socioeconomic status and a higher degree of disability than the general population. It is thus important to note that while these results were encouraging, a possible link between SSRIs and the development of ASD cannot be fully ruled out. Future studies could expand this cohort and explore other factors with a possible relationship to ASD.
Click to read the study, published in JAMA
Relevant Reading: Antidepressant Use During Pregnancy and the Risk of Autism Spectrum Disorder in Children
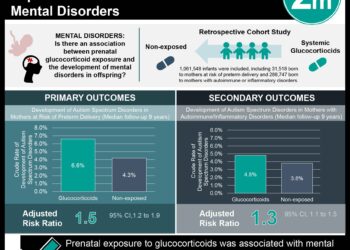
In-Depth [retrospective cohort]: This retrospective cohort study utilized health administrative data from patients receiving universal healthcare coverage in Ontario, Canada. Singleton children born between April 1, 2002 and March 31, 2010 to eligible mothers between ages 16 and 50 were considered. Exposure to serotonergic antidepressants was defined as two or more consecutive prescriptions of SSRI or serotonin norepinephrine reuptake inhibitors between conception and delivery. The unexposed group comprised of women without such usage during pregnancy or 90 days prior to conception. Follow-up occurred from a minimum 4 years to a maximum 10 years. ASD was defined by outpatient or in-hospital diagnosis by a licensed psychiatrist or pediatrician. To control for confounders, the study used inverse probability of treatment weighting based on the high-dimensional propensity score (HDPS).
A total of 35,906 singleton births (50.4% male) born at mean gestational age of 38.7 weeks were included in the study. The mothers were a mean age 26.7 years with 2,837 (7.9%) exposed to serotonergic antidepressants during pregnancy. Risk of ASD was significantly higher in exposed children compared with unexposed children (4.51 vs 2.03 unexposed per 1000 person-years) in both the crude and multivariable-adjusted analysis, but not after inverse probability of treatment weighting based on the HDPS (HR 1.61; 95%CI 0.997-2.59). A similar trend was observed in a sub-analysis by trimester of exposure and antidepressant class and type. However, for data restricted to women with mood and anxiety disorders, a significant relationship (after inverse probability of treatment weighting) was found for SSRIs as a class overall, and also specifically for antidepressant exposure in the second or third trimester. Risk of ASD did not differ significantly between exposed and unexposed siblings for both the crude and adjusted (for maternal age, parity, and calendar year of delivery) analysis.
Image: CC/Wiki
©2017 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.