Hypertonic saline may be effective in bronchiolitis
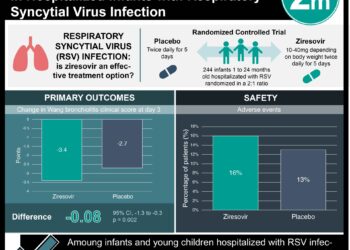
1. The use of hypertonic saline (HS) in bronchiolitis was associated with lower risk of hospital admission, shorter length of stay (LOS), and improved clinical severity scores.
2. The use of HS appears to be safe, with very few minor adverse events and no life-threatening events being reported.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Bronchiolitis is the leading cause of hospitalization for children under the age of 2 years and a significant contributor to emergency department and clinic visits. The primary therapy in most cases is supportive care, including the use of HS. This meta-analysis showed a statistically significant reduction in the risk of admission and inpatient LOS with the use of 3% saline in bronchiolitis. The use of HS in the inpatient setting was also associated with an improvement in clinical scores for illness severity. In addition, the use of HS appeared to be safe, with few adverse events being reported. While the study included a large cohort of patients, there was significant heterogeneity between the trials; furthermore, the included trials with high risk of selection bias showed significant effects on LOS and admission rates, whereas trials with lower risk of selection bias did not. Overall, these findings suggest that HS is appropriate and safe in the treatment of bronchiolitis, but its benefit may not be as large as previously believed.
Click to read the study, published today in Pediatrics
Relevant Reading: Nebulised hypertonic saline solution for acute bronchiolitis in infants.
In-Depth [systematic review and meta-analysis]: This study examined 24 trials selected from PubMed, the Virtual Health Library of the Latin American and Caribbean Center on Health Sciences Information and unpublished trials from ClinicalTrials.gov, resulting in 3209 included patients. Fifteen trials, with a total of 1956 patients, were examined for LOS. They showed a significantly shorter mean LOS among infants treated with HS compared to those treated with standard-of-care or normal saline, (mean difference -0.45 days; 95% CI -8.82 to -0.08, P=0.01). Seven trials, resulting in 951 patients, were pooled for assessment of HS on the risk of hospitalization. The pooled relative risk of admission with the use of HS was 0.80 (95% CI 0.67-0.96, P=0.01).The analysis of 5 trials, including 404 inpatients, showed significant improvement in clinical severity scores when using HS compared to standard of care (mean difference on day 3 of admission = -1.44, 95% CI -1.78 to -1.11, P=0.0001). Fourteen other trials (with a total of 1557 patients) reported no significant adverse events while 7 others reported few minor, self-resolving adverse events.
Image: PD
©2015 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.

![RSV positivity associated with reduced serious bacterial infection risk [Classics Series]](https://www.2minutemedicine.com/wp-content/uploads/2013/09/800px-Respiratory_syncytial_virus_01-350x250.jpg)