Liraglutide unable to improve post-hospitalization course in patients with advanced heart failure
1. This double-blinded, randomized controlled study found that liraglutide, a GLP-1 agonist, did no better than placebo in influencing post-hospitalization clinical stability.
2. There was no significant benefit of the drug in a subgroup of patients with type 2 diabetes.
Evidence Rating Level: 1 (Excellent)
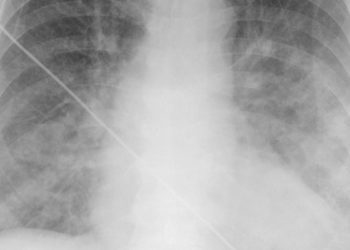
Study Rundown: Heart failure is a leading cause of hospitalization, and is attributed to abnormal cardiac metabolism. No current heart failure therapy directly targets these metabolic abnormalities. Thus, it was postulated that agents used to improve glucose metabolism could potentially improve the outcomes of patients with advanced heart failure. This double-blinded, randomized control trial (Functional Impact of GLP-1 for Heart failure Treatment: “FIGHT” study) tested the effects of a glucagon-like peptide 1 agonist, liraglutide, on improving clinical stability in patients with advanced heart failure after hospitalization. The study also analyzed subgroups of patients with and without type 2 diabetes. The results showed that that liraglutide did not improve clinical stability compared with placebo in these patients, nor did it alter left ventricular ejection fraction or N-terminal pro-B-type natriuretic peptide levels. There was no significant difference in deaths between groups. Subgroup analysis of patients with type 2 diabetes, showed equally non-significant results.
This double-blinded randomized control trial had multiple included many patients across several institutions. However, the study was limited by little power in subgroup analyses and a lack of functional metrics, like the 6-minute walk test. In conclusion, this study provides evidence that use of GLP-1 agonists such as liraglutide is not warranted in similar patients with advanced heart failure, nor in those patients with type 2 diabetes for the modulation of cardiac activity.
Click to read the study in JAMA
Relevant Reading: Incretin-based therapies for type 2 diabetes mellitus: effects on insulin resistance.
In-Depth [randomized controlled trial]: This multi-center, double blind, placebo controlled study aimed to quantify the effects of liraglutide, a GLP-1 agonist, on clinical stability in patients with advanced heart failure (with and without type 2 diabetes). It included 300 patients with advanced heart failure (AHF) and reduced left ventricular ejection fraction (LVEF). The primary outcome measure was a global rank score in which patients were ranked across three categories: time to death, time to re-hospitalization for AHF, and time-averaged proportional change in N-terminal pro-B natriuretic peptide (NT-proBNP) from baselined to 180 days. Higher values in this global rank score suggested better health and clinical stability. Another primary outcome measure was all-cause mortality.
In total, 154 patients received liraglutide, and 146, placebo. Fifty-nine percent of patients had type-2 diabetes. There was no significant difference in between-group differences for the global rank score (mean rank of 146 in the liraglutide group, 156 in the placebo group; Wilcoxon rank sum p = 0.31). In addition, there was no significant difference in the number of deaths (12% in liraglutide group, 11% in placebo group; hazard ratio 1.10; 95%CI 0.57-2.14; p = 0.78), nor the proportion of re-hospitalization for heart failure (41% in liraglutide group, 34% in placebo group; HR 1.30; 95%CI 0.89-1.88; p = 0.17). The time-averaged proportional change in NT-proBNP levels between groups was 1.52 (SD, 1.71) times baseline levels in the liraglutide group and 1.44 (SD, 1.22) times baseline levels in the placebo group (p = 0.94). Among the patients with type-2 diabetes, there was no statistically significant between-group differences in global rank score (mean rank 85 for liraglutide group and 94 for placebo group; p = 0.27), and the P-value for interaction was 0.60 for treatment based on type 2 diabetes status.
Image: PD
©2016 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.


![Reduced neuronal BRCA1 associated with decreased DNA repair in Alzheimer’s disease [PreClinical]](https://www.2minutemedicine.com/wp-content/uploads/2015/12/DNA_Furchen_edited-75x75.png)

