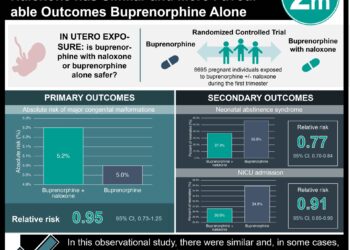
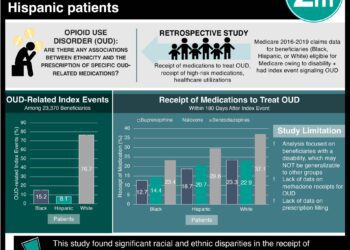
Nearly Half of All Pediatric Buprenorphine Exposures Result in Hospitalization
1. The majority of buprenorphine exposures in children 19 years and younger reported to the National Poison Data System (NPDS) were among children <6 years old and unintentional. However, the majority of exposures among adolescents aged 13 to 19 years were intentional and more commonly involved multiple substances.
2. Nearly half of reported exposures resulted in hospital admission. The most frequently reported clinical effects of exposure were drowsiness, vomiting, miosis, and respiratory depression.
Study Rundown: Buprenorphine is a prescription opioid used to treat opioid use disorder. Prescription rates are rising, as are reports of ingestions by children and adolescents. In this study, investigators sought to characterize the epidemiology of buprenorphine exposures by reviewing all incidents reported to the NPDS between 2007 and 2016. The majority of exposures were among children <6 years old and unintentional. The remainder of exposures were primarily among adolescents 13 to 19 years old. The majority of exposures among adolescents were intentional and more commonly involved multiple substances. Suspected suicide was the second most common reason for intentional exposure among adolescents, after misuse and/or abuse. Odds of hospital admission and serious medical were higher among children <6 years old, and among adolescents exposed to multiple substances.
The data were drawn from a passive surveillance system, which may be susceptible to under- or overreporting and ascertainment bias. The system also relies on self-report, though data is entered by qualified poison experts working at poison control centers. The study is strengthened by its national scope and the length of surveillance. For physicians, these results highlight the importance of informing patients prescribed buprenorphine, especially those who are caregivers of young children and adolescents, of the risks of accidental exposure and misuse.
Click to read the study, published today in Pediatrics
Relevant reading: Prescription opioid exposures among children and adolescents in the United States: 2000-2015
Study author, Gary A. Smith, MD, DrPH, speaks to 2 Minute Medicine: Director of the Center for Injury Research and Policy at Nationwide Children’s Hospital, Columbus, Ohio.
“Buprenorphine is an important medication for the treatment of opioid use disorder, but pediatric exposure can result in serious adverse outcomes. This study investigates the magnitude of the problem based on calls to US poison control centers over a 10-year period and describes steps that parents, health providers, and drug manufacturers can take to prevent pediatric buprenorphine exposures. In particular, drug manufacturers should use unit-dose packaging, often called blister packs, for all buprenorphine products to help prevent unintentional access and exposure by young children.”
In-Depth [passive surveillance study]: Investigators searched the NPDS of the American Association of Poison Control Centers for all reports of buprenorphine exposures among children 19 years and younger between 2007 and 2016. Study variables included age group (children <6, 6-12, and 13-19 years), reason for exposure, level of care at healthcare facility, and medical outcomes. Serious medical outcome was defined as death, life-threatening illness, significant residual disability or disfigurement, or moderate illness that involved some form of indicated treatment.
A total of 11 275 reports meeting study criteria were made to NPDS during the study period. Children <6 years old accounted for 86.1%, of all reports. Among all age groups, 89.2% of exposures were unintentional, 44.6% resulted in hospital admission, and 21.2% resulted in serious medical outcome, including 11 deaths. Among adolescents aged 13-19 years, 77.1% of exposures were intentional, 12.0% were used in a suspected suicide or suicide attempt, and 27.7% involved additional substances, most commonly benzodiazepines, ethanol, and marijuana. Adolescents exposed to multiple substances had higher odds of admission (OR: 4.74; 95%CI: 3.56-6.31) and serious medical outcome (OR: 2.97; 95%CI: 2.24-3.93) compared to adolescents exposed to buprenorphine alone. The most commonly reported clinical effects among all age groups were drowsiness (46.8%), vomiting (17.0%), miosis (12.6%), and respiratory depression (8.4%). All other effects were observed in less than 4% of cases.
Image: PD
©2018 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.