Older adults receiving recombinant influenza vaccine better protected against influenza
1. Older adults receiving recombinant influenza vaccine experienced fewer cases of polymerase chain reaction (PCR) confirmed influenza-like illness than patients receiving a similar inactivated influenza vaccine.
2. Greater efficacy of the recombinant vaccine was due to greater efficacy against the predominant influenza A strain of the 2014-2015 season, as efficacy against influenza B was similar between both vaccines.
Evidence Rating: 1 (Excellent)
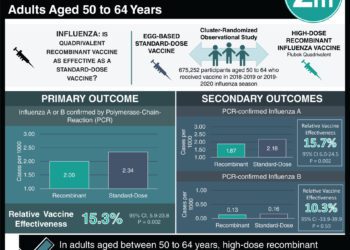
Study Rundown: As influenza is widespread and common, improvements to vaccines may provide great public health benefit. The recombinant influenza vaccine is produced faster than the traditional egg-grown vaccine and may be more effective. In the past influenza vaccine effectiveness in certain years has been low for older adults, a vulnerable group, making study of this demographic an important research target. This study looked to compare effectiveness of a quadrivalent recombinant influenza vaccine to an inactivated vaccine in adults age 50 and older.
This randomized controlled trial compared the two vaccines in older adults in outpatient centers during the 2012-2015 influenza season. Patients received one of the vaccines and followed-up to report any respiratory or systemic symptoms. Those experiencing symptoms returned to clinic to have a PCR assay performed assessing influenza types and subtypes. The study aimed to evaluate efficacy of the vaccines and safety. Patients receiving the recombinant vaccine were significantly less likely to have PCR-confirmed influenza-like illness than those receiving the inactivated vaccine. The recombinant vaccine was also more effective against influenza type A, though no difference between vaccine effectiveness was observed for influenza type B. Adverse revents were similar in occurrence with the two treatment groups.
Click to read the study, published in NEJM
Relevant Reading: Efficacy of high-dose versus standard-dose influenza vaccine in older adults
In-Depth [randomized controlled trial]: Between October 2014 and May 2015, 8963 patients were recruited, treated, and followed to assess efficacy of recombinant influenza vaccine (RIV4, n = 4474) versus an inactivated influenza vaccine (IIV4, n = 4489). Both vaccines contained 4 influenza strains recommended for the 2014-2015 influenza season. Eligible patients were 50 years old or greater without a significant acute illness. After vaccination, patients were asked by phone about various respiratory or systemic influenza-like symptoms. Patients with respiratory and systemic symptoms had PCR samples taken and assessed for influenza strain. The primary study endpoint was PCR confirmed influenza-like illness.
Patient characteristics of the RIV4 and IIV4 groups were similar. PCR confirmed influenza-like illness occurred in 2.2% (n = 96) of RIV4 patients and 3.2% (n = 138) of IIV4 patients, respectively. RIV4 vaccine efficacy was 30% greater than IIV4 (95% CI, 10-47; p = 0.006). RIV4 was also 36% more effective against influenza A (95% CI, 14-53; p = 0.003), though no difference in effectiveness was found against influenza B. Immunogenic response was also higher against the predominant A/H3N2 strain in RIV4 patients compared to those who received IIV4. Adverse events were experienced in 3.4% (n = 145) and 3.0% (n = 132) of RIV4 and IIV4 patients, respectively, though none of the events were considered related to the vaccines.
Image: PD
©2017 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.




![Adverse pregnancy outcomes associated with thrombophilias [Classics Series]](https://www.2minutemedicine.com/wp-content/uploads/2015/07/Classics-2-Minute-Medicine-e1436017941513-75x75.png)
