Ozanimod effective for induction and maintenance therapy of ulcerative colitis
1. Ozanimod treatment was shown to be more effective at inducing clinical remission than placebo for patients with ulcerative colitis.
2. Treatment with ozanimod was shown to have a significantly higher clinical response during induction and maintenance of ulcerative colitis.
Evidence Rating Level: 1 (Excellent)
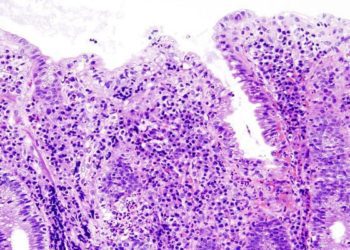
Study Rundown: Ulcerative colitis (UC) is a chronic disease, which causes chronic inflammation in the colonic mucosa. Previous therapies include aminosalicylates are moderately effective for patients with moderate but not severe disease and glucocorticoids, though they have been associated with long-term adverse effects. Ozanimod is a new treatment that has been proposed to serve as induction and maintenance therapy for patients with moderate to severe UC. However, there is a gap in knowledge as to understanding the effectiveness of ozanimod. This study found that the incidence of clinical remission was higher in patients on ozanimod therapy compared to the placebo. Additionally, the incidence of clinical response was also significantly higher in the ozanimod therapy group. This study was limited by the trial population potentially not being representative of the broader patient population as well as the lack of long-term data. Nevertheless, these study’s findings are significant, as they demonstrate that ozanimod is more effective than placebo for treatment of moderate to severely active UC.
Click to read the study in NEJM
Relevant Reading: Newer Biologic and Small-Molecule Therapies for Inflammatory Bowel Disease
In-Depth [randomized control trial]: This randomized controlled trial was a phase 3, multicenter study for a 52-week trial period. Patients who were between18 to 75 years of age, had moderate to severely active UC, and had received stable doses of oral aminosalicylates or glucocorticoids for at least 2 weeks before screening endoscopy were included in the study. Patients who did not have complete varicella-zoster vaccination, inability to provide informed consent, or presence of stool pathogens were excluded from the study. For the induction phase, there were 645 patients in cohort 1 who were randomized in a 2:1 ratio to either receive oral ozanimod (1 mg), daily or placebo, respectively. In cohort 2, 367 patients received open-label ozanimod, and this cohort was created to increase the number of patients with a response who would be available for randomization in the maintenance phase trial. For the maintenance phase, patients were randomized in a 1:1 ratio to either receive oral ozanimod (1 mg), daily or placebo, respectively. The primary endpoint was the percentage of patients with clinical remission at week 10 and week 52. Clinical remission was measured by the three-component Mayo score and defined as a rectal-bleeding sub-score of 0, a stool-frequency sub-score of 1 or less, and an endoscopy sub-score of 1 or less [all on scales from 0 (none) to 3 (most severe)]. Outcomes in the primary analysis were assessed via a closed, prespecified hierarchical testing procedure. Among the two groups, clinical remission was significantly higher in patients who received ozanimod therapy during induction (ozanimod, 18.4%; placebo, 6.0%; P<0.001) and during maintenance (ozanimod, 37.0%; placebo, 18.5%; P<0.001). Clinical response was also significantly higher in patients on ozanimod therapy compared to the placebo group both during induction (ozanimod, 47.8%; placebo, 25.9%; P<0.001) and maintenance (ozanimod, 60.0%, placebo, 41.0%; P<0.001). Overall, this study determined that the effectiveness of ozanimod as induction and maintenance therapy for moderate to severe UC is more effective than placebo treatment.
Image: PD
©2021 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.