Pazopanib plus paclitaxel may increase survival in platinum-resistant ovarian cancer
1. In patients with advanced, platinum-resistant ovarian cancer, combination therapy of a tyrosine kinase inhibitor, pazopanib, with paclitaxel significantly increased progression-free survival compared to paclitaxel alone.
2. Patients who received pazopanib plus paclitaxel suffered from higher rates of serious side effects including neutropenia, leucopenia, and hypertension.
Evidence Rating Level: 2 (Good)
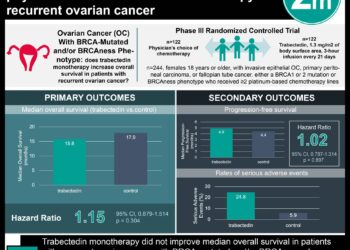
Study Rundown: Patients with ovarian cancer initially have excellent clinical response to platinum-based chemotherapy. Unfortunately, the majority of these patients develop disease recurrence or progression that is often refractory to platinum-based therapies. Within this patient cohort, anti-angiogenic therapy has been shown to have anti-tumor activity. In particular, pazopanib, a non-specific tyrosine kinase inhibitor of vascular endothelia growth factor (VEGF) receptors, has previously been shown to prolong progression-free survival in ovarian cancer patients compared to placebo. The purpose of this phase II trial is to assess the efficacy of pazopanib as an adjunct treatment modality in combiination with a taxane-based chemotherapy (paclitaxel). Overall, 74 patients with platinum-resistant or refractory ovarian cancer were randomized to weekly paclitaxel alone or pazopanib plus weekly paclitaxel. At the conclusion of the trial, the combination therapy group had a median progression-free interval of 6.35 months compared to 3.49 months for the paclitaxel alone group. Overall survival was longer in the combination therapy group, but did not reach significance. Unfortunately, patients in the combination group also suffered from higher rates of grade 3 and grade 4 adverse events including neutropenia, fatigue, leucopenia, hypertension, elevated liver transaminases, and anemia. The results of the Phase II trial demonstrated that the addition of pazopanib to weekly paclitaxel may improve progression-free survival compared to paclitaxel alone. However, this trial is limited by the open-label design and the relatively small sample size. Additional Phase III trials are indicated to confirm the result and to clarify the risk profile of this combination regimen.
Click to read the study in The Lancet Oncology
Click to read an accompanying editorial in The Lancet Oncology
Relevant Reading: Redefining the target: chemotherapeutics as antiangiogenics
In-Depth [randomized controlled trial]: The MITO 11 trial is a randomized, open-label, phase 2 trial of combination pazopanib and paclitaxel versus paclitaxel alone. Seventy-four patients from 11 hospitals in Italy with platinum-resistant or platinum-refractory ovarian cancer were included in the trial. Overall, 37 patients were randomized to combination pazopanib plus weekly paclitaxel and 37 were randomized to weekly paclitaxel alone. The primary endpoint was progression free survival with secondary endpoints of adverse effects and overall survival. After a median follow-up time of 16.1 months, the median progression-free survival was 6.35 months and 3.49 months for the combination therapy group and paclitaxel only group, respectively (HR: 0.42; 95% CI: 0.25-0.69; p=0.002). Median overall survival was 13.7 months and 19.1 months, respectively (HR: 0.60; 95% CI: 0.32-1.13; p=0.056). More patients in the combination group (51%) suffered from at least one grade 3 or 4 toxicity compared to the paclitaxel group alone (39%). The most common adverse events in the combination group were neutropenia (30%), fatigue (11%), leucopenia (11%) and hypertension (8%).
Image: PD
©2015 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.