Quick Take: Gentamicin compared with ceftriaxone for the treatment of gonorrhoea (G-ToG)
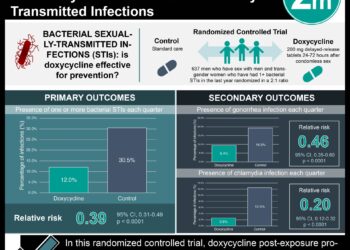
Gonorrhea is a common sexually transmitted infection associated with local inflammation causing genital pain, pelvic inflammatory disease, increased HIV acquisition risk, and other related complications. While ceftriaxone is the current first-line therapy, there is growing concern around emerging antimicrobial resistance, with limited evidence supporting other treatments. Gentamicin has been associated with cure rates ranging from 62% to 100%. However, studies reporting these rates have been poorly described with respect to their methodologies and therefore incur a high risk of bias. There have also been concerns surrounding gentamicin and gastrointestinal, ototoxic and nephrotoxic adverse effects. In this randomized non-inferiority trial, 720 adults were randomly assigned to receive a single intramuscular dose of either gentamicin 240 mg (n = 358) or ceftriaxone 500 mg (n = 362), in addition to a single 1 g dose of azithromycin, to assess the effectiveness of gentamicin as an alternative to ceftriaxone for treatment of gonorrhoea. A non-inferiority risk difference margin with a lower confidence limit of 5% was established. Researchers found that 299 (98%) participants in the ceftriaxone group compared to 267 (91%) in the gentamicin group achieved the primary outcome of clearance of N. gonorrhoeae infection after two weeks of treatment across all sites (genital, pharyngeal and rectal) (adjusted risk difference -6.4%, 95% CI -10.4% to -2.4%). Greater proportions of participants receiving ceftriaxone had clearance of pharyngeal infections (96% vs. 80%; adjusted risk difference -15.3%, 95% CI -24.0% to -6.5%) and rectal infections (98% vs. 90%; adjusted risk difference -7.8%, 95% CI -13.6% to -2.0%). Gentamicin performed better than ceftriaxone for genital gonorrhea, with clearance achieved in 98% vs. 94% of participants, respectively (adjusted risk difference -4.4%, 95% CI -8.7% to 0%). Side-effect profiles were similar between groups. This study therefore shows that while gentamicin plus azithromycin cannot be considered non-inferior to current standard therapy with ceftriaxone and azithromycin and is not suggested as a first-line regimen, its use may be appropriate for isolated genital infection, or for patients with ceftriaxone-related allergy, intolerance or resistance.
Click to read the study in Lancet
Image: PD
©2019 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.