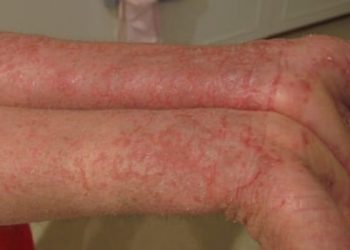
Roflumilast cream superior to vehicle cream in the treatment of plaque psoriasis – the DERMIS-1 and DERMIS-2 trials
1. Treatment of plaque psoriasis with roflumilast cream was associated with better clinical outcomes compared to vehicle cream after 8 weeks of treatment.
2. The incidence of serious adverse events was equal in the two treatment groups.
Level of Evidence Rating: 1 (Excellent)
Study Rundown: Roflumilast is a phosphodiesterase inhibitor (PDEi) which can be used in the management of respiratory disease such as chronic obstructive pulmonary disease. Recently, it has also been trialed as a topical formulation in the management of dermatological disease including plaque psoriasis. This study reports the results of two Phase 2b studies (DERMIS-1 and DERMIS-2) assessing roflumilast versus vehicle cream in the management of plaque psoriasis.
Data from 783 individuals was included in this study. In Trial 1 42.4% of the roflumilast-
treated and 6.1% of the vehicle-treated patients achieved the primary outcome at 8 weeks. At the same time point in Trial 2, 37.5% of the roflumilast patients and 6.9% of the vehicle patients had achieved the desired treatment response. Analysis of secondary outcomes demonstrated that roflumilast performed better with regards to achieving treatment response compared to the vehicle cream at all time points. The rate of adverse events in Trial 1 was 25.2% in the roflumilast group and 23.5% of patients in the vehicle group. In Trial 2 the rate of adverse events in the intervention and control groups were 25.9% and 18.4%, respectively. The rate of serious adverse events was 0.7% in all trial groups except for the roflumilast group in Trial 2, who experienced zero adverse events.
This study by Lebwohl et al summarizes the results of two randomized controlled trials of roflumilast cream versus placebo for the management of plaque psoriasis. The study showed a significant improvement in clinical status following treatment with roflumilast compared to the vehicle cream at all time points, and no difference in the rate of adverse events. The results of this trial are convincing, given the randomized design which does well to account for confounding and the strong treatment effect demonstrated in the roflumilast group. A limitation of the two trials is the lack of follow-up data beyond 8 weeks.
Click here to read this study in JAMA
Click to read an accompanying editorial in JAMA
Relevant reading: Roflumilast foam 0.3% treatment of seborrheic dermatitis is effective and safe and improves patient quality of life: results from a phase 2 study
In Depth [randomized controlled trial]: The results of two trials are reported in this article: Trial 1 was a 12-week trial of 331 patients and Trial 2 was a 52-week trial. Patients were eligible if they were aged 2 years or above and had been diagnosed with plaque psoriasis for at least 6 (adults) or 3 (children) months. The primary outcome was measured by the Investigator Global Assessment of success (IGA) in terms of clearance of the psoriatic plaques. Specifically, treatment success was defined as nearly/all clear, or improvement by at least 2 IGA stages at week 8 of treatment. Patients were randomized in a 2:1 fashion to treatment with either 0.3% roflumilast cream or vehicle cream; randomization was stratified by study site, baseline IGA score and presence of intertriginous lesions. Participants were blinded to the nature of the intervention.
Trial 1 included data from 388 participants and Trial 2 included data from 395 participants. Baseline characteristics and demographic information were balanced between the two groups in each trial. In Trial 1, 42.4% of the roflumilast-treated and 6.1% of the vehicle-treated patients achieved the primary outcome at 8 weeks. The mean difference was 39.6% (95% confidence interval 32.3-46.9%). After 8 weeks in Trial 2, 37.5% of the roflumilast patients and 6.9% of the vehicle patients had achieved the desired treatment response. The mean effect difference was 28.8% (95% confidence interval 20.8-36.9%). The number needed to treat for improvement with roflumilast was 2.8 (2.3-3.4) in Trial 1 and 3.3 (2.6-4.3) in Trial 2. There were no significant differences in rates of adverse events between the two groups.
Image: PD
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.