#VisualAbstract: Continued enzalutamide after progression of metastatic prostate cancer improves progression-free survival
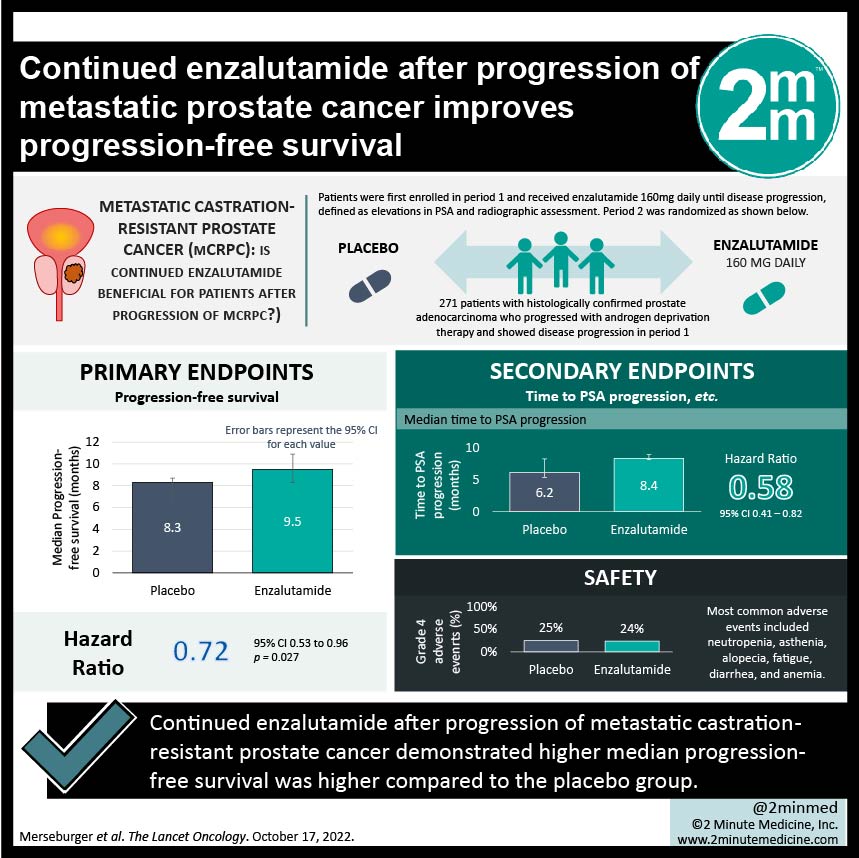
1. The median progression-free survival was higher in the enzalutamide vs the placebo group (9.5 months vs 8.3 months)
2. The enzalutamide group had more fatigue and asthenia.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Metastatic castration-resistant prostate cancer (mCRPC) has been treated with effective first-line therapies docetaxel (chemotherapy) with neo/adjuvant enzalutamide (androgen receptor inhibitor) based on past trials including AFFIRM and PREVAIL. Yet what remains unclear is the choice of therapy should there be disease progression with the above treatment plan. This randomized phase 3b trial investigates treatment for patient with mCRPC who had disease progression after therapy with enzalutamide. Patients were randomized into either receiving enzalutamide or placebo, in addition to a standard dose of docetaxel and prednisolone. The primary endpoint was progression-free survival and the secondary endpoints included time to PSA progression, PSA response, objective response rate, and more. It was found that the median progression-free survival was 9.5 months in the enzalutamide vs 8.3 months in the placebo group. Median time to PSA progression was 8.4 months in the enzalutamide group vs 6.2 months in the placebo group. Objective response rates were similar in both groups (41% vs 39% respectively). Grade 3 or higher adverse events also occurred at similar rates for both groups and the most common adverse events included neutropenia, asthenia, alopecia, fatigue, diarrhea, and anemia. Limitations to this study was that it was not powered for overall survival, selected a low risk-population as the eligible patients had higher functional status and had endocrine-responsive cancers. Strengths of this study included its large sample size, and its randomization. Overall, in patients with mCRPC who have disease progression despite enzalutamide therapy, continuing enzalutamide with docetaxel has shown to be effective.
Click to read the study in Lancet Oncology
Relevant Reading: Increased Survival with Enzalutamide in Prostate Cancer after Chemotherapy
In-Depth [randomized controlled trial]: PERSIDE was a multinational double blinded randomized placebo-controlled phase 3b trial which enrolled patients with histologically confirmed prostate adenocarcinoma who progressed with androgen deprivation therapy. Patients were first enrolled in period 1 and received enzalutamide 160mg daily until disease progression, defined as elevations in PSA and radiographic assessment. Those who had disease progression were randomized in period 2 into either receiving enzalutamide 160mg daily or placebo, in addition to a standard dose of docetaxel and prednisolone. 688 patients were enrolled in period 1, and 271 patients had disease progression and were randomly assigned in period 2. The median follow-up time was 8.1 months in the enzalutamide group (136 patients) and 6.3 months in the placebo group (135 patients). Median progression-free survival was 9.5 months (95%CI, 8.3-10.9) in the enzalutamide group and 8.3 months (95%CI, 6.3-8.7) in the placebo group, which resulted in HR 0.72 (95%CI, 0.53-0.96, p=0.027). Disease progression occurred in 74% of the enzalutamide group vs 76% in the placebo group. Median time to PSA progression was 8.4 months (CI95%, 8.2-9.0) in the enzalutamide group vs 6.2 months (CI95%,5.4-8.3) in the placebo group, HR 0.58 (95%CI, 0.41-0.82). The objective response rates were 41% (8CR/13PR) in the enzalutamide group vs 39% (7CR/16PR) in the control group. Grade 3/grade 4 adverse events occurred in 38%/24% vs 37%/25% in the enzalutamide compared to placebo, respectively. The most common adverse events included neutropenia (highest amount of grade 3/4 events), asthenia, alopecia, fatigue, diarrhea, and anemia. Overall, continuing treatment with enzalutamide/docetaxel was shown to be effective when compared to placebo/docetaxel in patients with mCRPC.
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.