#VisualAbstract: Eculizumab in Aquaporin-4–Positive Neuromyelitis Optica Spectrum Disorder
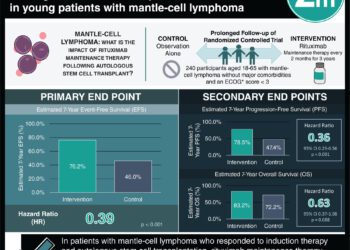
1. In this randomized control trial of neuromyelitis optica spectrum disorder (NMOSD) patients with aquaporin-4 antibodies (AQP4-IgG), those randomized to receive the eculizumab complement inhibitor experienced a lower relapse rate over 24 months than placebo treated patients.
2. Eculizumab patients more commonly experienced upper respiratory tract infections (URI) and headaches.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Neuromyelitis optica spectrum disorder (NMOSD) is a recurrent autoimmune disorder mainly characterized by optic neuritis and myelitis. Rituximab is an approved treatment to prevent relapses but is not effective for all patients. AQP4 is a protein in the central nervous system which a majority of NMOSD patients have IgG antibodies to, and the AQP4-IgG complex leads to complement activation facilitating relapses. Eculizumab is a complement inhibitor thought to be efficacious at ultimately reducing NMOSD relapse events. The PREVENT trial (Prevention of Relapses in Neuromyelitis Optica) evaluated a primary outcome of first adjudicated relapse in placebo or eculizumab treated patients and found relapses occurred less in treated patients. Eculizumab treated patients had disability scores that trended towards improvement from baseline, though they did experience more URIs and headaches than placebo patients. One death due to pulmonary empyema occurred in an eculizumab patient.
This randomized control trial suggests notable efficacy of eculizumab in preventing NMOSD relapses in a major subpopulation of patients. While the efficacy data is marked, the study is limited by the difficulties in interpreting an NMOSD relapse which ultimately stopped the trial.
Click to read the study in NEJM
©2019 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc