#VisualAbstract: Encorafenib, Binimetinib, and Cetuximab in BRAF V600E–Mutated Colorectal Cancer
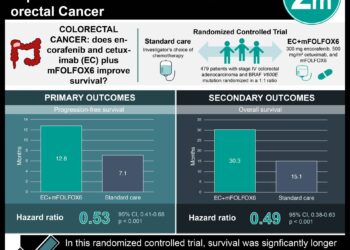
1. A phase 3 randomized clinical trial showed that combined therapy using a BRAF inhibitor (encorafenib), EGFR monoclonal antibody (cetuximab) and a MEK inhibitor (binimetinib) led to longer overall disease survival in patients with BRAF V600E mutated metastatic colorectal cancer as compared to control groups who received a single agent.
2. Doublet therapy with encorafenib-cetuximab was also shown to have longer overall disease survival as compared to control.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Colon cancer patients with the BRAF V600E mutation have been shown to have poorer prognoses. Approximately 10% of patients with metastatic colon cancer have this mutation, and standard chemotherapy regimens have shown to have limited success. A novel BRAF inhibitor, encorafenib, was studied in this trial in conjunction with EGFR monoclonal antibody treatment (cetuximab) and MEK inhibitor treatment (binimetinib) to evaluate whether combined treatment would result in longer disease survival as compared to the standard therapy available to patients with BRAF V600E mutated colorectal cancer. Overall, researchers found that combined triple therapy led to longer overall survival as compared to control. They also found that combined therapy with two experimental agents also led to longer overall survival as compared to control. These results are promising in creating new novel agents for treating metastatic colorectal cancer in this particular subset of patients, however more research is warranted so that further differences in treatment outcomes between triplet therapy and doublet therapy can be investigated.
Click here to read the study in NEJM
©2019 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.