1. Patients with peripheral artery disease who received a drug-coated device did not face an increased risk of death compared to those who received an uncoated device at any point during the 2+ year follow-up.
2. This finding appeared to be independent of disease severity, although the wide confidence interval was suggestive of a potential difference by subgroup.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Endovascular intervention appears to be effective in treating peripheral artery disease but is associated with a considerable risk of postprocedural restenosis. Coating balloons and stents with antiproliferative agents such as paclitaxel has been shown to reduce this risk, but the effects of drug-coated devices on patient-oriented endpoints and long-term outcomes remain unclear. The Swedish Drug Elution Trial in Peripheral Arterial Disease (SWEDEPAD) was originally designed to assess the impact of drug-coated devices on quality of life and incidence of amputation; after a recent meta-analysis revealed an increased late risk of death following application of these devices, patient recruitment was halted and an unplanned interim analysis of all-cause mortality was conducted. After a mean follow-up period of two and a half years, the percentages of patients in the drug-coated and uncoated groups who had died were found to differ by less than a single point. Deaths generally occurred at a continuous rate and did not vary between groups at any time point; further, this trend seemed to be consistent across both the chronic limb-threatening ischemia and intermittent claudication subgroups. This study had a lengthy follow-up and no patient attrition, but it was limited by an open-label design and may have been underpowered to detect differences within subgroups. Additionally, low-dose devices were used more frequently than high-dose devices, which could have attenuated any effects associated with paclitaxel. Overall, the results from this randomized trial supported the safety of paclitaxel-coated devices over the short to medium term.
Click here to read the study, published in NEJM
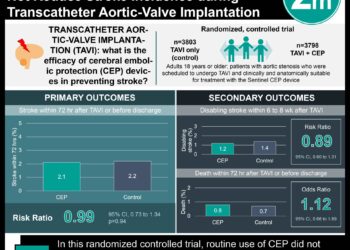
In-Depth [randomized controlled trial]: In this multicenter, single-blind trial with recruitment from November 2014 until December 2018, 2289 adults with symptomatic peripheral artery disease caused by flow-limiting stenosis or occlusion in infrainguinal arteries were placed into one of two cohorts according to severity (chronic limb-threatening ischemia vs. intermittent claudication). These groups were then randomly assigned in a 1:1 ratio during endovascular balloon angioplasty or stenting (after guide wire placement but before dilation) to receive treatment with either a paclitaxel-coated device or an uncoated device. During a mean follow-up of 2.49 years (2.34 for chronic group and 2.77 for intermittent group), a total of 574 patients (25.1%) died. Of these, 293 belonged to the drug-coated group (1480 patients) and 281 belonged to the uncoated group (809 patients), corresponding to a hazard ratio (HR) of 1.06 (95% confidence interval [CI], 0.92 to 1.22) and a mortality rate per 100 patient-years of 10.4% and 9.8%, respectively. No between-group difference was detected in either the chronic subgroup (33.4% [249 patients] vs. 33.1% [243 patients]; HR, 1.04; 95% CI, 0.90 to 1.21) or the intermittent subgroup (10.9% [44 patients] vs. 9.4% [38 patients]; HR, 1.18; 95% CI, 0.72 to 1.93).
Image: PD
©2021 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.