#VisualAbstract: Torsemide does not provide additional decrease in mortality compared to furosemide among patients hospitalized for heart failure
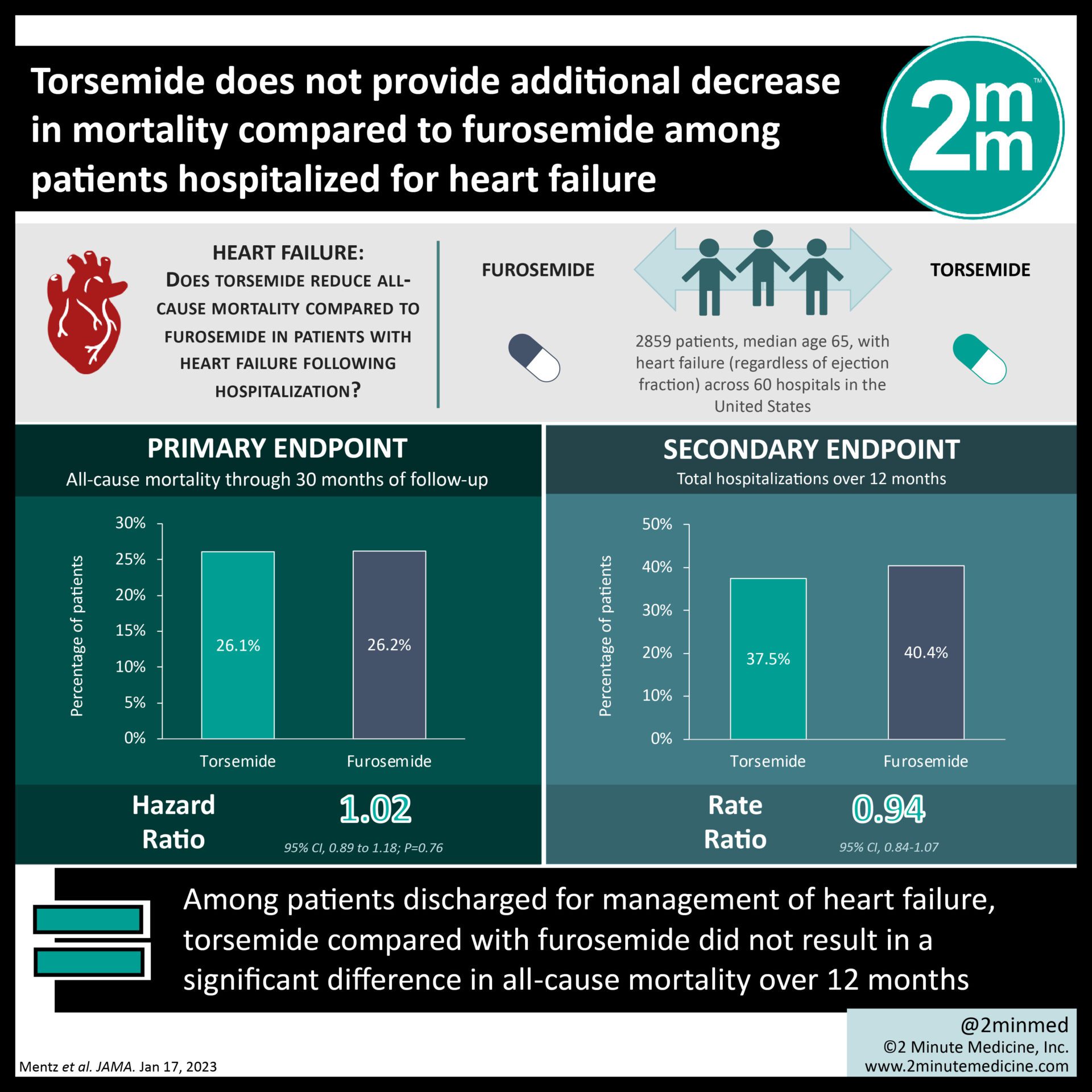 1. In this randomized clinical trial, among 2859 patients, death occurred in 26.1% of patients randomized to torsemide and 26.2% of patients randomized to furosemide over a median follow-up period of 17.4 months, with no significant difference between groups.
1. In this randomized clinical trial, among 2859 patients, death occurred in 26.1% of patients randomized to torsemide and 26.2% of patients randomized to furosemide over a median follow-up period of 17.4 months, with no significant difference between groups.
2. Rates of hospitalizations in the torsemide and furosemide groups were 37.5% and 40.4%, respectively.
Evidence Rating Level:1 (Excellent)
Study Rundown: Heart failure (HF) is commonly characterized by symptoms of congestion and volume overload that can present as dyspnea and edema. Current guidelines recommend the use of diuretics for the treatment of congestion in symptomatic patients with HF. Furosemide is the most commonly prescribed diuretic for HF, however, recent clinical data suggest potential benefits of torsemide when compared when furosemide. The objective of the TRANSFORM-HF (Torsemide Comparison with Furosemide for Management of Heart Failure) trial was to compare the effectiveness of torsemide with furosemide in patients hospitalized with HF. A total of 2859 participants hospitalized with HF were recruited between 2018 and 2022, with follow-up through 30 months for death and 12 months for hospitalizations. Patients were randomized to receive either torsemide (n=1431) or furosemide (n=1428). The main outcome was all-cause mortality, and secondary outcomes included all-cause hospitalization and total hospitalizations assessed over 12 months. Death occurred in 26.1% of patients in the torsemide group, and 26.2% of patients in the furosemide group. 12 months post-randomization, all-cause mortality or all-cause hospitalization occurred in 47.3% of patients in the torsemide group and 49.3% of patients in the furosemide group. When compared with furosemide, torsemide did not result in a significant difference in all-cause mortality over 12 months. A major strength of this trial was that over 30% women were recruited for both intervention groups. Interpretation of these findings may be limited, however, due to the loss to follow-up, participant crossover and non-adherence.