Artemisinin-based antimalarials may be safe and efficacious in pregnant women
1. All four artemisinin-based treatment groups had cure rates of over 94%. Artemether-lumefantrine was statistically less efficacious than the other three regimens however was only less efficacious by 2%.
2. Rates of serious adverse events were not statistically significant across the four treatment groups.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Little information is known about the efficacy and toxicity of new anti-malaria treatment options in pregnant women. Given the risk of maternal anemia and infant mortality in pregnant women with malaria, it is important to study the new Artemisinin-based combination therapies in pregnant women to ensure their safe use. This study aimed to determine the safety and efficacy profile of four Artemisinin-based antimalarial treatments African pregnant women.
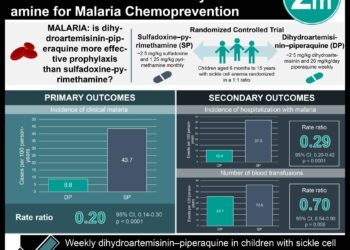
All treatment groups provided polymerase-chain-reaction (PCR)-adjusted cure rates above 94%. However, statistically, the artemether-lumefantrine group did poorer as compared to amodiaquine-artesunate, mefloquine-artesunate or dihydroartemisinin-piperaquine. There were no significant differences in serious adverse events across all treatment groups. Strengths of this study include a large sample size of an often-underrepresented study population. Limitations noted include the lack of double-blinding and comparative placebo control group for assessment of efficacy.
Click to read the study, published today in NEJM
Click to read an accompanying editorial in NEJM
Relevant Reading: Malaria in pregnancy
In-Depth [randomized controlled trial]: This randomized, open-label controlled trial was conducted between June 2010 and August 2013 in seven sites in four sub-Saharan African countries (Burkina Faso, Ghana, Malawi, Zambia). The study population included pregnant women in the second or third trimester who had Plasmodium falciparum monoinfection, hemoglobin >70g/L and no other serious illnesses. These women were randomly assigned to one of four treatment groups: artemether-lumefantrine, amodiaquine-artesunate, mefloquine-artesunate or dihydroartemisinin-piperaquine. The primary endpoint of interest was PCR-adjusted cure rates at day 63 and safety outcomes. The primary statistical analysis was a per-protocol method.
A total of 3428 pregnant women were enrolled and randomized to the four treatment groups: artemether-lumefantrine (881 women), amodiaquine-artesunate (843), mefloquine-artesunate (849) or dihydroartemisinin-piperaquine (855). The PCR-adjusted cure rates in the per-protocol analysis was 94.8% in artemether-lumefantrine, 98.5% in amodiaquine-artesunate, 96.8% in mefloquine-artesunate and 99.2% in the dihydroartemisinin-piperaquine treatment group. There was no significant difference in treatment efficacy among amodiaquine-artesunate, mefloquine-artesunate or dihydroartemisinin-piperaquine however the cure rate was significantly lower in the artemether-lumefantrine group. There was no significant difference in the rate of serious adverse events or birth outcomes across all treatment groups.
Image: PD
©2016 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.