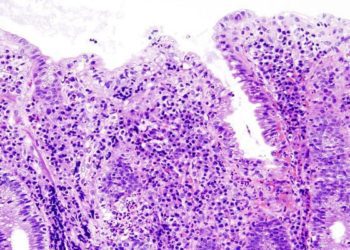
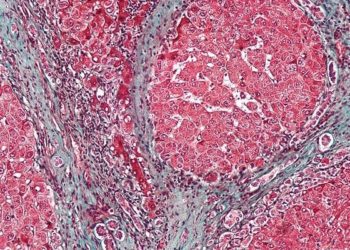
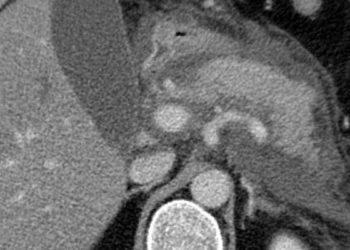
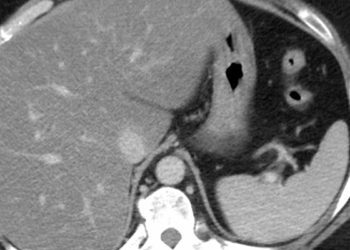
The incidence of ocular manifestations is higher in patients with Crohn’s disease compared to ulcerative colitis
1. Among patients with adult-onset inflammatory bowel disease (IBD), the incidence of ocular manifestations was 2.9 per 1000 person-years. 2....