Difelikefalin associated with reductions in itch intensity and improvements in itch-related quality of life
1. In this double-blind phase 3 trial involving patients undergoing hemodialysis who had moderate-to-severe pruritus, a clinically significant decrease in itching intensity was noted in a greater percentage of patients in the difelikefalin group than in the placebo group.
2. On average, improvements in itching-related quality of life were also greater in the difelikafalin group.
Evidence Rating Level: 1 (Excellent)
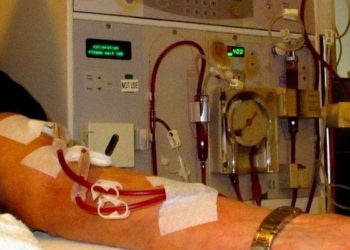
Study Rundown: Pruritus affects the majority of patients undergoing hemodialysis and is associated with reduced quality of life and increased risk of death. While the pathogenesis of uremic pruritus is not yet fully understood, a number of hypotheses have been posited, including endogenous opioid system imbalance. Difelikefalin is a peripherally restricted, selective kappa opioid receptor agonist that has produced clinically significant benefits in phase 2 trials involving patients undergoing hemodialysis who had moderate-to-severe pruritus. This phase 3 trial found that difelikefalin resulted in dramatic reductions in itching intensity as well as significant improvements in itch-related quality of life, corroborating the findings of earlier-stage trials. Adverse events, although high in both groups, occurred slightly more frequently in the treatment group, with diarrhea, dizziness, and vomiting being the most common. Dysphoria and hallucinations were notably absent, indicating a possible safety advantage of peripherally restricted kappa opioid agonists versus mu opioid and centrally acting kappa opioid receptor agonists. While the robustness of this data was confirmed through sensitivity analyses, these findings have limited generalizability due to specific eligibility criteria and a relatively short follow up.
Click here to read the study in NEJM
Relevant Reading: Treatment of Uremic Pruritus: A Systematic Review
In-Depth [randomized controlled trial]: In this multicenter, double-blind, phase 3 trial, 378 patients undergoing hemodialysis who had moderate-to-severe pruritus were stratified according to history of prespecified medical conditions and concomitant antipruritic use, and randomly assigned in a 1:1 ratio to receive either difelikefalin at a dose of .5 mcg/kg or placebo three times per week for 12 weeks. At baseline, patients scored similarly on the 24 hour Worst Itching Intensity Numerical Rating Scale (WI-NRS), averaging 7.1±1.4 in the difelikefalin group and 7.3±1.6 in the placebo group. At week 12, a significantly greater proportion of patients in the treatment group showed an improvement (decrease) of at least 3 points from baseline in the weekly mean score on the 24-hour WI-NRS versus placebo (49.1% vs. 27.9%; relative risk, 1.65; 95% confidence interval, 1.26 to 2.14; P<0.001). The treatment group also experienced greater improvements in quality of life according to multidimensional metrics such as least-squares mean change from baseline in 5-D itch scale total score (-5.0±0.3 vs. -3.7±0.3) and least-squares mean change from baseline in Skindex-10 total score (-17.2±1.3 vs. -12.0±1.2) (all P-values <0.001). Of the five dimensions of itch, four (distribution, duration, degree, and direction) displayed a between-group difference in favor of difelikefalin. Adverse events leading to discontinuation of treatment were more common in the difelikefalin group (7.9% vs. 4.8%).
Image: PD
©2020 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.