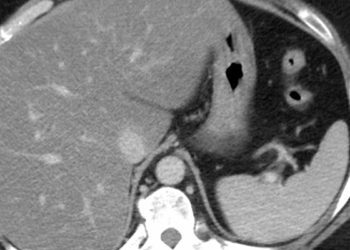
Guselkumab is safe and effective in patients with moderate-to-severe Crohn’s disease
1. The Guselkumab group demonstrated significantly greater clinical and endoscopic responses versus placebo.
2. Serious adverse events were comparable, with no treatment-related deaths.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Despite the availability of biological therapies, many patients with Crohn’s disease experience suboptimal disease control. This randomized controlled trial aimed to evaluate the efficacy and safety of intravenous induction followed by subcutaneous maintenance therapy with guselkumab over 48 weeks in adults with active Crohn’s disease. The primary outcome of this study was clinical response at week 12 and clinical remission at week 48, while a key secondary outcome was clinical response at week 12 and endoscopic response at week 48. According to study results, guselkumab was significantly more effective than placebo in achieving clinical remission and endoscopic response by week 48. Although this study was well done, it was limited by the lack of long-term follow-up data.
Click to read the study in The Lancet
Relevant Reading: Risankizumab versus Ustekinumab for Moderate-to-Severe Crohn’s Disease
In-depth [randomized controlled trial]: Between Jan 8, 2020, and Oct 20, 2023, 1823 patients were screened for eligibility across 257 sites in 40 countries. Included were patients aged ≥ 18 years with moderate-to-severe active Crohn’s disease. Altogether, 1021 patients (508 in GALAXI-2 and 513 in GALAXI-3) were included in the final analysis. The primary outcome of clinical response at 12 weeks and clinical remission at 48 weeks was achieved significantly more often with both guselkumab regimens compared to placebo (GALAXI-2: 55% in the 200 mg maintenance group and 49% in the 100 mg maintenance group vs. 12% in placebo; GALAXI-3: 48% and 47% vs. 13%; p<0·0001). The secondary outcomes of clinical response at 12 weeks and endoscopic response at 48 weeks also showed superiority of both guselkumab dosing regimens over placebo in both studies (GALAXI-2: 38% and 39% vs. 5%; GALAXI-3: 36% and 34% vs. 6%; p < 0.0001). Findings from this study suggest that guselkumab is effective and well-tolerated in patients with moderate to severe Crohn’s disease.
Image: PD
©2025 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.