Imetelstat linked with selective telomerase activity response in myelofibrosis
1. The Imetelstat response rate was significantly higher in patients without ASXL1 mutations, with SF3B1 mutations, or with U2AF1 mutations. Further studies to understand the precise mechanism of action of this molecule are required.
2. Myelosuppression was the most significant side effect of this treatment. Hepatic and hematologic side effects included thrombocytopenia, neutropenia, anemia, and elevated liver enzyme levels.
Evidence Rating: 2 (Good)
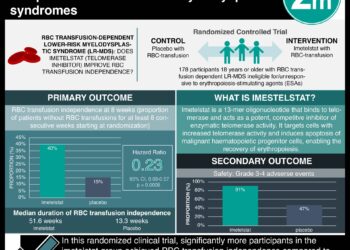
Study Rundown: Currently, myeloproliferative neoplasm-associated myelofibrosis (MF) can only be treated with allogeneic stem-cell transplantation. But this treatment is associated with a high rate of treatment-related death and complications, including chronic graft-versus-host disease. Telomerase maintains the telomere length in rapidly dividing cells and is active in most cancer cells but not normal somatic tissue. Imetelstat is a 13-mer lipid-conjugated oligonucleotide that has been shown to inhibit telomerase activity in various cancer cell models. This study collected preliminary information on the therapeutics and safety of imetelstat in patients with MF. It included a total of 33 patients with MF (median age = 67 years of age). Response rates to imetelstat was significantly higher in patients without the ASXL1 mutation compared to those with the mutation. Furthermore, patients with mutation in SF3B1 or U2AF1 demonstrated a significantly higher rate of complete response compared to patients without a mutation in these genes. The most significant side effect of the treatment was myelosuppression. Other side effects included grade 4 thrombocytopenia (21%), grade 4 neutropenia (18%), grade 3 anemia (52%), and grade 1 or 2 elevated liver-enzyme levels during the treatment protocol (bilirubin by 45%, ALP 52%, AST by 58%, ALT by 27%).
Click to read the study in NEJM
Relevant Reading: Targeting telomerase-expressing cancer cells
In-Depth [prospective cohort]: This primary correlative study was an investigator-driven, single-center study. Eligibility criteria included the conventional definition of primary MF or post-polycythemia vera or post-essential thrombocythemia MF, high-risk or intermediate-2-risk disease according to the Dynamic International Prognostic Scoring System (DIPSS) Plus; a platelet count of at least 50×10 per liter; an absolute neutrophil count of at least 1×109 per liter; AST and ALP levels up to 2.5x UL; a total bilirubin level of up to 3.0 mg per deciliter; and a serum Cr level of up to 3.0 mg per deciliter. A total of 33 patients with MF were enrolled in the study. The median age of the patients were 67 years and majority of them were men (67%). Partial or complete remission occurred in 21% of the patients with a median duration of response of 18 months. Response rates were significantly higher in patients without an ASXL1 mutation compared to those with an ASXL1 mutation (32% vs. 0%; p=0.07). Patients with SF3B1 or U2AF1 had a 38% rate of complete response compared to 4% of patients without the mutation (p=0.04). Telomere length was not a determinant factor in the response rates that were observed (p=0.34).
Image: PD
©2015 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.