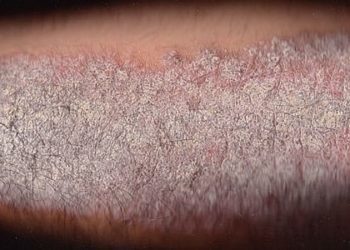
Ixekizumab may be safe and effective for chronic plaque psoriasis
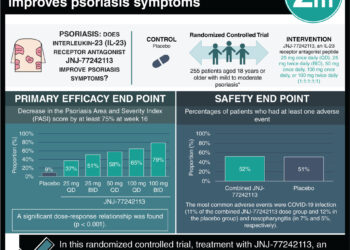
1. The use of 120mg ixekizumab in a phase-III, open-label extension (OLE) trial showed improved Psoriasis Area and Severity Index (PASI) outcomes in patients with chronic plaque psoriasis.
2. Ixekizumab was not associated with major cardiac events or deaths in this study.
Evidence Rating Level: 2 (Good)
Study Rundown: Psoriasis is a common immune-mediated skin condition involving pro-inflammatory cytokines such as interleukin-17A. The use of ixekizumab, an interleukin-17A monoclonal antibody, has been associated with improved skin symptoms in a previous phase-II randomized control trial (RCT). This study was an OLE of the aforementioned RCT to test the efficacy and safety of ixekizumab. The PASI score, a measure of severity and extent of psoriasis was used to measure treatment response. Ixekizumab was shown to improve PASI in patients with chronic plaque psoriasis. Additionally, though adverse events were reported, including depression, suicide attempt, rectal cancer, atherosclerosis, and nephrolithiasis, no major cardiac events or deaths were observed. Though this study showed positive results, the lack of a control group limits the drug efficacy and safety comparability to other conventional psoriatic drugs.
Click to read the study in JAAD
Relevant Reading: Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis
In-Depth [placebo-controlled trial]: This study was an OLE trial on a previous phase-II RCT on the use of ixekizumab for the treatment of chronic plaque psoriasis. A total of 120 patients participated in this trial and 103 (86%) finished 52 weeks of ixekizumab, 120mg treatment. The PASI criteria (comparing treatment results with baseline lesions) were used to measure drug efficacy. Overall, 92/120 patients had a PASI75, 81/120 had a PASI90, and 58/120 had achieved a PASI100 by the 52nd week of treatment. Patients were 58% males, 93% Caucasian, 93% had been previously treated for psoriasis, and the average age was 47 years. An adverse event incidence rate of 0.06 for serious adverse events per patient-year during the OLE trial was observed.
More from this author: Video-based behavioral intervention benefits clinical skin examinations, Biofilm-producing staphylococci occlude eccrine sweat ducts in atopic dermatitis, Initial primary invasive or in situ melanoma increases risk of subsequent invasive melanoma, Various factors impact quality of life in patients with chronic pruritus, Primary melanoma regression may not be associated with reduced metastasis
Image: Wiki/Heilman
©2012-2014 2minutemedicine.com. All rights reserved. No works may be reproduced without expressed written consent from 2minutemedicine.com. Disclaimer: We present factual information directly from peer reviewed medical journals. No post should be construed as medical advice and is not intended as such by the authors, editors, staff or by 2minutemedicine.com. PLEASE SEE A HEALTHCARE PROVIDER IN YOUR AREA IF YOU SEEK MEDICAL ADVICE OF ANY SORT.


![Novel biodegradable sirolimus-eluting stents non-inferior to durable everolimus-eluting stents [BIOSCIENCE trial]](https://www.2minutemedicine.com/wp-content/uploads/2014/09/Taxus_stent_FDA-e1607803635904-75x75.jpg)

