Lentiviral gene therapy prevents functional deterioration in cerebral adrenoleukodystrophy patients
1. In this prospective cohort study, in boys with diagnosed cerebral adrenoleukodystrophy, it was found that lentiviral gene therapy containing ABCD1 complementary DNA permitted survival free from major functional disability.
2. Patients treated with the gene therapy did not experience graft versus host disease (GVHD).
Evidence Rating Level: 1 (Excellent)
Study Rundown: In X-linked adrenoleukodystrophy, pathogenic variations in ABCD1 impair the function of the peroxisomal transporter ATP-binding cassette domain 1 (ABCD1), leading to the accumulation of saturated very-long-chain fatty acids in the body. Approximately 30% of individuals with this condition develop cerebral adrenoleukodystrophy, characterized by inflammation and demyelination of white matter. As a result, the disease is associated with significant cognitive and neurological decline, often leading to early death. Previous research has suggested that elivaldogene autotemcel (eli-cel) gene therapy comprising autologous hematopoietic stem cells transduced with Lenti-D lentiviral vectors with ABCD1 complementary DNA may help prevent disease progression. This study aims to evaluate the efficacy and safety of this treatment and describe its long-term effects, employing an initial 24-month study followed by a subsequent 13-year follow-up period. Most patients survived without any of the defined major functional disabilities and maintained baseline neurologic function scores. While no cases of GVHD were documented, conditioning before eli-cel infusion resulted in numerous serious adverse events. Overall, this study has limitations, including a small sample size, excluding individuals with more extensive disease based on their Loes score, and a relatively short follow-up period. Despite these limitations, the findings suggest that eli-cel gene therapy may be a viable treatment option for preventing disease progression in a subset of patients with cerebral adrenoleukodystrophy.
Click here to read the study in NEJM
Relevant Reading: Hematopoietic Stem-Cell Gene Therapy for Cerebral Adrenoleukodystrophy
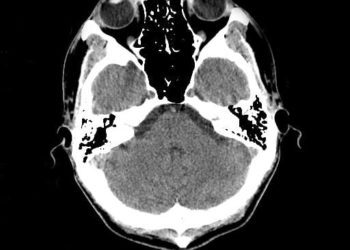
In-Depth [prospective cohort study]: This prospective cohort study investigated the efficacy and safety of eli-cel gene therapy as a treatment for cerebral adrenoleukodystrophy. Patients were eligible if they had a confirmed diagnosis of cerebral adrenoleukodystrophy through biochemical and genetic testing, exhibited characteristic gadolinium enhancement on brain MRI, had a neurologic function score of 0 or 1, and had a Loes score between 0.5 and 9. Exclusion criteria included having an HLA-matched sibling willing to donate cells for hematopoietic stem cell transplantation (HSCT), the standard of care for cerebral adrenoleukodystrophy. Between October 2013 and April 2019, 32 patients were enrolled in the study, which included an initial 24-month period followed by a 13-year follow-up. One primary outcome of the study was patient survival without any of the six identified major functional disabilities by the end of the initial 24-month study. Of the 32 patients, 30 survived until the completion of the 24-month study, and of these, 29 met this endpoint (95% confidence interval [CI], 75-98). The primary safety outcome was acute (grade II or higher) or chronic GVHD by 24 months. At this time point, no cases of GVHD were reported in any of the patients, contrasting with the 18-31% typical occurrence in HSCT patients. However, the overall safety profile of the treatment remains to be fully established; 69% of patients experienced serious adverse events, and 25% experienced serious infections. Of the surviving patients, 87% showed no gadolinium enhancement by month 24 (95% CI, 69-96) and 80% achieved a Loes score consistent with their baseline (95% CI, 61-92). Furthermore, 97% of patients demonstrated stable neurological function scores (95% CI, 83-100). Overall, these findings suggest that eli-cel gene therapy may be a viable treatment option for certain patients with cerebral adrenoleukodystrophy.
Image: PD
©2024 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.