Long-term use of rituximab associated with lower rates of AAV relapse
1. In this randomized controlled trial involving patients with antineutrophil cytoplasmic antibody-associated vasculitis in complete remission, administration of rituximab for an additional 18 months was associated with a lower likelihood of relapse versus placebo.
2. Although the vast majority of patients in both groups reported at least 1 adverse event, major relapse-free survival was 100% in the treatment group.
Evidence Rating Level: 1 (Excellent)
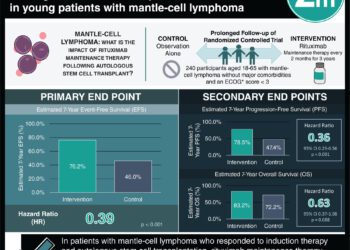
Study Rundown: Current FDA recommendations for the treatment of antineutrophil cytoplasmic antibody-associated vasculitis (AAV) involve glucocorticoids and rituximab infusion therapy. While the MAINRITSAN (Maintenance of Remission Using Rituximab in Systemic ANCA-Associated Vasculitis) and MAINRITSAN2 trials clearly demonstrated the efficacy of rituximab in maintaining remission throughout the 18-month treatment period, nearly half of the patients went on to experience relapse within 3 years of discontinuation. This trial investigated the benefit of extending the treatment schedule for an additional 18 months, finding that none of the patients who continued to receive rituximab had a major relapse 28 months after re-randomization compared to 13% of the placebo group. No significant differences were observed in the incidence of minor relapse, the progression of organ damage, or quality of life. Adverse events and serious adverse events occurred at similar rates in both groups. The primary limitation of this study was that only patients who had completed the MAINRITSAN2 trial were eligible to be recruited, resulting in a relatively small and homogenous sample population. Nonetheless, the results of this study support the prolonged use of rituximab to prevent relapse in patients with AAV.
Click here to read the study, published today in Annals of Internal Medicine
Relevant Reading: Rituximab versus Azathioprine for Maintenance in ANCA-Associated Vasculitis
In-Depth [randomized controlled trial]: In this multicenter, double-blind randomized control trial, 97 patients who completed the MAINRITSAN2 trial without any major relapses and were in complete remission according to the Birmingham Vasculitis Activity Score were randomly assigned in a 1:1 ratio to receive either biannual 500-mg rituximab infusions or placebo for 18 months. Of these patients, 68 had granulomatosis with polyangiitis and 29 had microscopic polyangiitis. At 28 months, relapse-free survival was 96% (95% CI, 91% to 100%) in the rituximab group versus 74% (95% CI, 63% to 88%) in the placebo group (hazard ratio, 7.5; P = 0.008). While 6 patients in the placebo group experienced major relapses (3 renal and 3 pulmonary flares), both relapses in the treatment group were minor. Antineutrophil cytoplasmic antibody (ANCA) positivity was observed in 50% of patients who had a relapse and only 15% of those who did not. Over 90% of patients in both groups had an adverse event of any severity, and infectious serious adverse events occurred in 12% of the treatment group and in 9% of the control group. At the end of the study, the rituximab group had a mean gamma globulin level of 6.6 g/L versus a concentration of 7.9 g/L in the placebo group (P < 0.001).
Image: PD
©2020 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.