Neoadjuvant FOLFOX compared to Chemoradiotherapy in Locally Advanced Rectal Cancer
1. Patients who were randomized to neoadjuvant FOLFOX vs standard chemoradiation had similar disease-free and overall survival rates.
2. There were more severe (grade ≥3) adverse events in the FOLFOX group compared to the standard chemoradiation group.
Evidence Rating Level: 1 (Excellent)
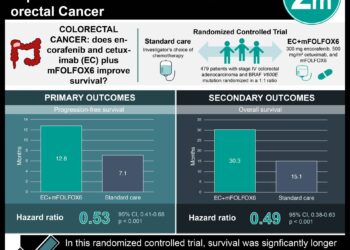
Study Rundown: Pelvic chemoradiotherapy for locally advanced rectal cancer reduces the risk of disease recurrence in the pelvis, but it has negative effects on quality of life and physical function. Some studies have suggested that preoperative pelvic chemotherapy with fluorouracil sensitization and postoperative chemotherapy with the FOLFOX regimen improved disease-free survival in patients with rectal and colon cancer. This non-inferiority trial investigated if neoadjuvant FOLFOX is as effective as neoadjuvant chemoradiotherapy alone for locally advanced rectal cancer patients. The primary endpoint was noninferiority for disease-free survival (DFS), and secondary endpoints included overall survival (OS), local recurrence, pathological response rates, and toxic effects. Five-year DFS was 80.8% in the FOLFOX group and 78.6% in the chemoradiotherapy group, with HR 0.92. Five-year OS was 89.5% vs 90.2% in the FOLFOX vs chemoradiotherapy group, with HR 1.04. Local recurrence at 5 years was 1.8% and 1.6%, respectively with HR 1.18. With regards to safety, severe (grade ≥3) adverse events occurred in 41.0% of the FOLFOX group vs 22.8% in the chemoradiotherapy group. The most frequent grade 3 or higher toxic effects in the FOLFOX group were neutropenia, pain, and hypertension; vs the chemoradiotherapy group had lymphopenia, diarrhea, and hypertension. The strengths of the study included its long follow-up period and adequate sample size. The limitations of this study included the exclusion of high-risk patients. Overall, this study found that neoadjuvant FOLFOX chemotherapy with selective chemoradiotherapy was a viable alternative to the standard approach for locally advanced rectal cancer and may represent an approach with fewer long-term radiotherapy complications.
Click to read the study in NEJM
Relevant Reading: Neoadjuvant Chemotherapy Without Routine Use of Radiation Therapy for Patients With Locally Advanced Rectal Cancer: A Pilot Trial
In-Depth [randomized controlled trial]: This multicenter, unblinded, noninferiority trial randomized adults with untreated, pathologically confirmed locally advanced rectal cancer staged T2-T3 into the FOLFOX group (6 cycles, 585 patients) vs the chemoradiotherapy group (50.4 Gy together with fluorouracil or capecitabine, 543 patients) before surgery. In both groups, adjuvant FOLFOX was suggested but not mandated. The patients in the FOLFOX group received chemoradiotherapy if their tumour shrank less than 20%, or who were unable to complete at least 5 cycles of FOLFOX. The median follow-up was 58 months. In the FOLFOX group, 9.1% received neoadjuvant chemoradiotherapy. Five-year DFS was 80.8% (95%CI, 77.9 to 83.7) in the FOLFOX group and 78.6% (95%CI, 75.4 to 81.8) in the chemoradiotherapy group, with HR 0.92 (90.2%CI, 0.74 to 1.14, p=0.005 for noninferiority). Five-year OS was 89.5% vs 90.2% in the FOLFOX vs chemoradiotherapy group, with HR 1.04 (95%CI, 0.74 to 1.44). Local recurrence at 5 years was 1.8% and 1.6%, respectively with HR 1.18 (95%CI, 0.44 to 3.16). Postoperatively, 74.9% of the FOLFOX group and 77.9% of the chemoradiotherapy group received adjuvant chemotherapy. With regards to safety, severe (grade ≥3) adverse events occurred in 41.0% of the FOLFOX group (12 weeks of treatment) vs 22.8% in the chemoradiotherapy group (5.5 weeks of treatment). The most frequent grade 3 or higher toxic effects in the FOLFOX group were neutropenia (20.3%), pain (3.1%), and hypertension (2.9%); vs the chemoradiotherapy group had lymphopenia (8.3%), diarrhea (6.4%), and hypertension (1.7%). Overall this study found that neoadjuvant FOLFOX chemotherapy with selective chemoradiotherapy was a viable alternative to the standard approach for locally advanced rectal cancer.
Image: PD
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.