Nintedanib may be effective in slowing the progression of interstitial lung disease
1. In this phase 3 trial, patients with fibrosing lung disease who received nintedanib experienced a much slower rate of decline in forced vital capacity compared to those who received placebo.
2. Patients in the nintedanib group were more likely to experience gastrointestinal discomfort and elevations in liver enzymes versus placebo.
Evidence Rating Level: 1 (Excellent)
Study Rundown: The tyrosine kinase inhibitor nintedanib has been suggested to delay the progression of a number of lethal lung disorders including idiopathic pulmonary fibrosis and systemic sclerosis-associated interstitial lung disease. Given the many clinical and pathophysiological commonalities between such conditions, it has been hypothesized that a broad range of other progressive fibrosing disorders may be similarly receptive to treatment with this drug. This study found that, compared to the placebo group, patients with fibrosing interstitial lung disease who received nintedanib experienced a significantly smaller decrease in forced vital capacity and a more favorable change in score on the King’s Brief Interstitial Lung Disease questionnaire after 52 weeks. The rates of adverse events of any severity were similar between groups, but those receiving nintedanib were more likely to experience an adverse event leading to permanent dose reduction or discontinuation of treatment. Additionally, over two thirds of patients in the nintedanib group reported having diarrhea compared to only one fourth of patients in the placebo group. Strengths of this study included patient diversity, stratification according to fibrotic pattern, and use of a Hochberg procedure in statistical analysis. However, due to the breadth of the inclusion criteria, it remained difficult to determine the relative efficacy of nintedanib in the treatment of specific conditions since results may not have applied equally to all patient subsets.
Click here to read the study in NEJM
Relevant Reading: The role of tyrosine kinases in the pathogenesis of idiopathic pulmonary fibrosis
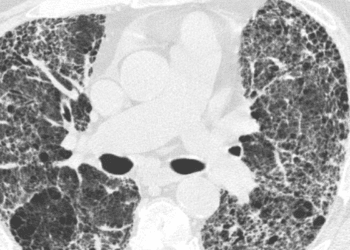
In-Depth [randomized controlled trial]: This randomized, double-blind, placebo-controlled, parallel-group trial was conducted at 153 sites in 15 countries and recruited 663 adults with any fibrosing interstitial lung disease affecting more than 10% of lung volume as shown on high-resolution computed tomography. Patients were also required to demonstrate downward trends in forced vital capacity (FVC), respiratory symptoms, and extent of fibrosis. After stratification by imaging pattern (usual interstitial pneumonia (UIP) like or other), patients were randomized to receive either oral nintedanib at a dose of 150 mg twice daily or placebo. The overall adjusted rate of decline in FVC was -80.8 mL/year in the nintedanib group versus -187.8 mL/year in the placebo group (between-group difference, 107.0 ml; 95% confidence interval [CI], 65.4 to 148.5; P<0.001). This difference was even greater in patients with a UIP-like pattern, with those receiving nintedanib experiencing a rate of decline of -82.9 mL/year versus −211.1 mL/year in those receiving placebo (between-group difference, 128.2 ml; 95% CI, 70.8 to 185.6; P<0.001). In the overall population, the adjusted change in the total score on the King’s Brief Interstitial Lung Disease questionnaire after 52 weeks was 0.55 points in the nintedanib group and −0.79 points in the placebo group (between-group difference, 1.34 points; 95% CI, −0.31 to 2.98). Finally, while the percentages of patients with adverse events remained similar between groups, those in the nintedanib group were more likely to experience an event leading to either dose reduction (33.1% vs. 4.2%) or discontinuation of treatment (19.6% vs. 10.3%).
Image: PD
©2020 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.


![The ABCD2 score: Risk of stroke after Transient Ischemic Attack (TIA) [Classics Series]](https://www.2minutemedicine.com/wp-content/uploads/2013/05/web-cover-classics-with-logo-medicine-BW-small-jpg-350x250.jpg)


