Quick Take: Assessment of use of arteriovenous graft vs. arteriovenous fistula for first-time permanent hemodialysis access
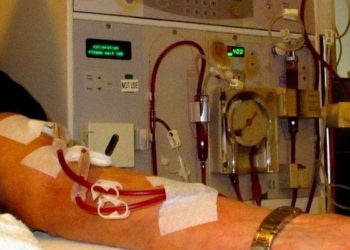
For patients with end-stage renal disease, the type of hemodialysis access selected has a direct effect on complication rates and associated healthcare costs. Typically, morbidity and mortality is lower with arteriovenous fistula (AVF) as compared to arteriovenous graft (AVG) or central venous catheter use; as such, clinical practice guidelines for hemodialysis access recommend the use of AVF whenever feasible. In this cohort study, Medicare Carrier clams (2016-2017) for all patients undergoing initial permanent hemodialysis access placement (n=85,320) were used to explore contemporary rates of AVF versus AVG use and determine physician characteristics associated with high AVG use. Researchers found that 77.9% of patients undergoing first-time hemodialysis placement had an AVF, compared to 22.1% of patients receiving an AVG. Among the 2397 surgeons who performed more than 10 procedures per year, the median surgeon level AVG use rate was 18.2%; however there were 498 surgeons (20.8%) who had an AVG use rate exceeding 34%. Upon adjusting for patient characteristics, surgeon factors that were independently associated with AVG use included more than 30 years of clinical practice when compared to surgeons with 21-30 years of clinical practice (OR 0.85, 95% CI 0.75 to 0.96), metropolitan setting (OR 1.25, 95% CI 1.02 to 1.54) and vascular surgery specialty (OR 0.77, 95% CI 0.69 to 0.86) versus general surgery specialty. This study therefore shows that as many as 20% of surgeons have an AVG use rate above recommended best practice guidelines. Sharing benchmarked performance data with surgeons may be an actionable next step in shifting towards AVF with respect to hemodialysis access surgery.
Click to read the study in JAMA Surgery
Image: PD
©2019 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.


![Novel biodegradable sirolimus-eluting stents non-inferior to durable everolimus-eluting stents [BIOSCIENCE trial]](https://www.2minutemedicine.com/wp-content/uploads/2014/09/Taxus_stent_FDA-e1607803635904-350x250.jpg)


