Varenicline and buproprion more effective than varenicline alone for tobacco abstinence
Image: PD
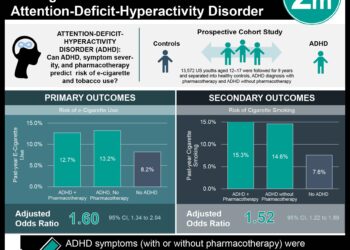
1. The combination of varenicline and buproprion for 12 weeks was more effective than varenicline alone at achieving sustained abstinence from tobacco.
2. There was no statistically significant difference between varenicline and buproprion sustained release and varenicline alone in prevalence of smoking the past 7 days at 12 or 26 weeks. At 52 weeks, there was no difference in abstinence two weeks after a target quit date or smoking in the past seven days.
Evidence Rating Level: 1 (Excellent)
Study Rundown: There are several non-nicotine pharmacotherapies for tobacco dependence. Currently, there is interest in exploring combination therapy with existing tobacco dependence therapies. This study is a randomized, blinded, placebo-controlled trial of varenicline and buproprion sustained release (SR) versus varenicline and placebo. 506 participants were randomized and treated for 12 weeks and followed for 40 weeks after cessation of therapy. The combination therapy increased prolonged abstinence, defined as no smoking in the two weeks after the target quit date at 12 and 26 weeks. However, the authors found no difference between 7-day point smoking prevalence at 12 weeks or 26 weeks. At week 52, there was no significant difference between either of the outcomes. A strength of this study is the use of carbon monoxide (CO) level to biochemically confirm reports of abstinence. One weakness of the study is the fact that most participants were Caucasian. Ultimately, a longer trial with a more diverse population will be needed to elucidate the role of combination therapy for tobacco dependence.
Click to read the study, published today in JAMA
Relevant Reading: Varenicline and bupropion sustained-release combination therapy for smoking cessation
In-Depth [randomized, blinded, placebo-controlled study]: This study looked at varenicline plus buproprion SR versus varenicline plus placebo for tobacco dependence. 506 individuals were treated for 12 weeks and followed for an additional 40 weeks. After 12 weeks of treatment, 53.0% of the combination therapy individuals achieved prolonged abstinence while only 43.2% of individuals achieving prolonged abstinence with monotherapy (OR 1.49, 95% CI 1.05-2.12, p=0.03 and OR 1.36 95% CI 0.95-1.93, p=0.09, respectively). At 26 weeks of treatment, 36.6% of the participants on combination therapy achieved prolonged abstinence compared to 27.6% individuals on monotherapy (OR 1.52, 95% CI 1.04-2.22, p=0.03 and OR 1.32, 95% CI 0.91-1.91, p=0.14, respectively). At one year, 30.9% of the combination group achieved prolonged abstinence compared to 24.5% varenicline only group (OR 1.39, 95% CI 0.93-2.07, p=0.11 and OR 1.40, 95% CI 0.96-2.05, p=0.08).
By Jeffrey Cohen and Brittany Hasty
More from this author: Most physicians point to others to control healthcare costs, QRS morphology and duration associated with cardiac resynchronization outcomes, Repeat bone mineral density testing may not improve prediction of fracture outcomes, Risk-reduction counseling with HIV testing may not decrease rates of sexually transmitted infections, Autoantibodies may precede symptom onset in Sjögren’s Syndrome
©2012-2014 2minutemedicine.com. All rights reserved. No works may be reproduced without expressed written consent from 2minutemedicine.com. Disclaimer: We present factual information directly from peer reviewed medical journals. No post should be construed as medical advice and is not intended as such by the authors, editors, staff or by 2minutemedicine.com. PLEASE SEE A HEALTHCARE PROVIDER IN YOUR AREA IF YOU SEEK MEDICAL ADVICE OF ANY SORT.