#VisualAbstract: Association of Genetic Variants in NUDT15 With Thiopurine-Induced Myelosuppression in Patients With Inflammatory Bowel Disease
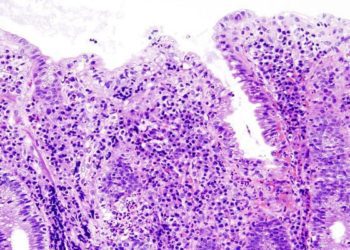
1. In this case-control study of patients of European ancestry with inflammatory bowel disease (IBD), variants in NUDT15 were associated with an increased risk of thiopurine-induced myelosuppression (TIM).
2. There were no sex-based differences in these genetic variants nor were there any differences based on IBD type.
Evidence Rating Level: 2 (Good)
Study Rundown: Thiopurine-induced myelosuppression (TIM) is an adverse drug reaction that occurs in approximately ten percent of patients who receive thiopurines for the management of inflammatory bowel disease (IBD). Currently, the FDA recommends genetic screening for the thiopurine S-methyltransferase (TPMT) gene prior to the initiation of thiopurine-based therapy. In this case-control study, IBD patients of European ancestry that had both the TPMT and the nudix hydrolase 15 (NUDT15) genes had an increased likelihood for developing TIM. For patients with the TPMT genetic variant, they were three times as likely to develop TIM while those with the NUDT15 genetic variant were thirty-eight times as likely to develop TIM. When patients had both genetic variants, they were significantly more likely to develop neutropenia, be hospitalized, and to have received granulocyte-colony stimulating factor rescue therapy. There were no sex-based differences in these genetic variants nor were there any differences based on IBD type.
While this study highlights some important applications of precision medicine, its conclusions are currently limited to patients of European ancestry. In order to more broadly apply these findings, these genetic variants should be studied in additional patient populations in order to increase the generalizability of these findings.
Click to read the study in JAMA
©2019 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.