#VisualAbstract: Voclosporin shows clinical promise for treatment of active lupus nephritis
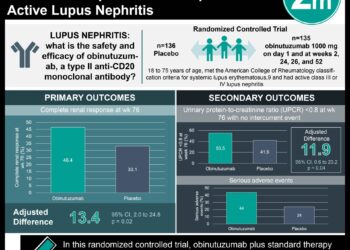
1. A significantly greater proportion of patients in the voclosporin group achieved complete renal responses after 52 weeks compared to the placebo group.
2. The safety profile between the voclosporin group and placebo group were comparable with 21% of patients in both groups experiencing serious adverse events.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Nearly a quarter of patients with lupus nephritis develop renal failure in ten years. Despite recent clinical trials on promising biologic drugs, an effective oral medication would have additional benefits, such as a lower cost and convenience. Voclosporin is a novel calcineurin inhibitor which holds many pharmacokinetic advantages over its older CNI counterparts, which have long been used to improve renal response rates. This phase III drug trial conducted in 27 countries randomized 357 individuals to either voclosporin or placebo, with both groups receiving standard mycophenolate mofetil and low-dose steroids. After 52 weeks, the voclosporin group had significantly increased rates of complete renal response compared to placebo. The safety profile of both drugs was found to be comparable, with the same percentage of participants experiencing adverse events. A limitation of this study includes its short duration, as this is a preliminary study to be followed for an additional two years. Nonetheless, this phase 3 drug trial provides promising evidence for the efficacy and safety of voclosporin.
Click to read the study in the Lancet
Relevant Reading: Two-year, randomized, controlled trial of Belimumab in lupus nephritis
In-Depth [randomized control trial]: This double-blind, randomized phase 3 drug trial recruited 357 patients with active lupus nephritis in 142 clinics across 27 countries between April 13, 2017 to Oct 10, 2019. 179 patients were assigned to receive voclosporin and 178 received a placebo. All patients were given mycophenolate mofetil and low-dose oral steroids. The primary outcome investigated was renal response at week 52 assessed by the independent Clinical Endpoints Committee. Significantly more patients on voclosporin (41%) had a complete renal response compared to placebo (23%; odds ratio 2.65, p<0.0001). This statistical significance in favour of voclosporin was maintained for all secondary outcomes, such as partial response at 52 weeks or complete response at 24 weeks. Safety was determined by occurrence of serious adverse events, which were comparable at 21% of patients in both groups. Analysis was done by intention-to-treat and drop-outs were seen in the voclosporin (9%) and placebo (17%) group.
Image: PD
©2021 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.