Whole-genome sequencing more accurate than cytogenetic analysis in myeloid cancers
1. Whole-genome sequencing for myeloid cancers, compared to standard cytogenic analysis, was able to more effectively risk-stratify cytogenic cases.
2. Inconclusive cytogenetic analysis cases were classified into risk groups by whole-genome sequencing, thereby, providing a prognosis.
Evidence Rating Level: 2 (Good)
Study Rundown: Genetic abnormalities are important for the classification of myeloid cancers such as acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS). The cancers are typically identified through cytogenetic analysis such as karyotyping. Whole-genome sequencing detects all types of mutations and may reveal more cryptic mutations. For patients with cytogenic results that were unavailable at diagnosis, whole-genome sequencing was able to risk-stratify patients that correlated with outcomes such as overall survival. Limitations to the study include implementation of the testing are the laboratory investments required to perform this analysis. Furthermore, the cost of sequencing varies and will be one of the large determining factors of the health-economic benefit. The authors do demonstrate an efficient platform to perform whole-genome sequencing for myeloid cancers. Additional health-economic analyses will be needed before there is clear evidence of the benefit of whole-genome sequencing in cancer care.
Click here to read the study in the NEJM
Relevant Reading: Economic impact of genomic diagnostics for intermediate‐risk acute myeloid leukaemia
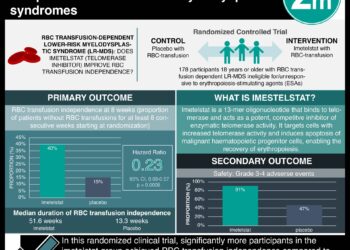
In-Depth [prospective cohort]: This mixed retrospective and prospective study enrolled 235 patients at Washington University at St. Louis. Patients with known or suspected AML or MDS diagnoses were included in the study. Patients with diagnosed myeloid cancer that were not AML or MDS were excluded from the study. This study was a head-to-head comparison of whole-genome sequencing with conventional cytogenic and FISH analyses. The authors designed an approach to whole-genome sequencing termed ChromoSeq. Whole-genome sequencing detected all recurrent translocations and copy-number alterations that had been identified by cytogenetic analysis. The median processing time was 5.1 days. From the prospective cohort, 16% changed the risk category based on the whole-genome analysis. In 27 patients with AML who could not be assigned a risk group based on cytogenetic analysis, patients were risk-stratified based on mutations identified by whole-genome sequencing. The intermediate or favourable risk group (n = 21) had longer overall survival than the adverse group (n = 6) (hazard ratio, 0.25; 95% confidence interval, 0.09 to 0.94). Overall, this study demonstrates the emerging feasibility of comprehensive mutational analysis in cancer care which will require significant infrastructural investments to efficiently apply in clinical care.
Image: PD
©2021 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.

![The ABCD2 score: Risk of stroke after Transient Ischemic Attack (TIA) [Classics Series]](https://www.2minutemedicine.com/wp-content/uploads/2013/05/web-cover-classics-with-logo-medicine-BW-small-jpg-350x250.jpg)