2 Minute Medicine Rewind January 6, 2020
1. Immune-related adverse events are associated with a longer recurrence-free survival in patients with stage III melanoma treated with pembrolizumab.
Evidence Rating Level: 1 (Excellent)
Immune-related adverse events (irAEs) occur frequently in patients treated with immune checkpoint inhibitors (ICIs). Retrospective studies and small prospective studies have reported an association between irAEs and improved outcomes in patients with melanoma and lung cancer, suggesting that irAEs may indicate increased drug activity. However, these associations require validation in larger prospective studies with adequate statistical methods. In this secondary analysis of a randomized controlled trial of 1,019 adults with stage III melanoma treated with either pembrolizumab or placebo, 1,011 patients commenced treatment and were studied to investigate the association between irAEs and recurrence-free survival (RFS). At baseline, 61.5% of patients were men, and 62.7% of patients were aged 50 years or older. The median follow-up period was 15 months (IQR 13 to 17 months). Researchers found that the RFS was longer in the pembrolizumab arm compared with the placebo arm (HR 0.56, 98.4% CI 0.43 to 0.74), consistent with the reported main analysis. The incidence of grade 1 or higher irAEs at both 3 months and 15 months was greater in the pembrolizumab group than in the placebo group (3 months: pembrolizumab 19.4%, 95% CI 16.1% to 23.0%; placebo 4.0%, 95% CI 2.5% to 6.0%, 15 months: pembrolizumab 37.4%, 95% CI 33.2% to 41.6%; placebo 9.0%, 95% CI 6.7% to 11.7%; HR 4.95, 95% CI 3.58 to 6.85, p<0.001). The most common irAEs were endocrine disorders and vitiligo. Of those who experienced an irAE, 17.3% patients in the pembrolizumab arm and 13.3% in the placebo arms discontinued treatment due to an irAE. The occurrence of an irAE was associated with a longer RFS in the pembrolizumab arm (HR 0.61, 95% CI 0.39 to 0.95, p=0.03) but not in the placebo arm (HR 1.37, 95% CI 0.82 to 2.29, p=0.21). Moreover, compared with the placebo arm, the reduction in the hazard of recurrence or death in the pembrolizumab arm was greater after an irAE than without or before an irAE (HR 0.37, 95% CI 0.24 to 0.57 vs. HR 0.61, 95% CI 0.49 to 0.77, respectively, p=0.03). Finally, systemic steroid use for irAEs was associated with a higher estimated HR than no steroid use (HR 0.50, 95% CI 0.23 to 1.07 vs. HR 0.34, 95% CI 0.21 to 0.56, respectively). In summary, this study suggests that irAEs are associated with a longer recurrence-free survival in patients with stage III melanoma treated with pembrolizumab.
1. There is no significant difference in 30-day all-cause mortality between radial and femoral access for percutaneous coronary intervention (PCI) following ST-segment elevation myocardial infarction (STEMI).
Evidence Rating Level: 1 (Excellent)
Primary percutaneous coronary intervention (PCI) is the standard of care for achieving reperfusion in patients with ST-segment elevation myocardial infarction (STEMI). However, the procedure is not without substantial risk, mostly related to exposure to potent antiplatelet and anticoagulant therapies. Radial access is gaining popularity based on studies demonstrating a better safety profile than femoral access, and two recent trials have suggested that radial access is associated with lower mortality after PCI. However, this finding remains a topic of debate. In this randomized controlled trial, 2,292 patients with STEMI referred for primary PCI were assigned to either radial access or femoral access to study the primary outcome of 30-day all-cause mortality. The baseline characteristics of the two study groups were equivalent. In terms of medications and procedural choices, all patients were administered a loading dose of a P2Y12 platelet inhibitor before cardiac catheterization. During PCI, bivalirudin was used in 88.1% of patients in the radial access group and 92.4% of the patients in the femoral access group. The median interval between administration of lidocaine and balloon inflation/device was 2 minutes longer in the radial access group (radial median 13 minutes, IQR 10 to 17 minutes; femoral median 11 minutes, IQR 9 to 14 minutes; p<0.001). Furthermore, the median (IQR) fluoroscopy time was also significantly longer in the radial access group than in the femoral access group (9.4 minutes, 6.5 to 13.5 vs. 8.2 minutes, 6.0 to 12.5, p<0.001). Ultimately, the trial was terminated early due to a lower-than-expected rate of the primary outcome. At study termination, researchers found that there was no significant difference in all-cause 30-day mortality between the two groups (radial access: 1.5%; femoral access: 1.3%; RR 1.15, 95% CI 0.58 to 2.30, p=0.69). Moreover, there were no significant differences in any of the secondary outcomes, including stroke, reinfarction, stent thrombosis, and bleeding within 30 days. Limitations of this study include the exclusion of patients treated with fibrinolytic therapy and patients prescribed oral anticoagulants. Overall, this study suggests that there is no survival benefit of using radial access for primary PCI, but further studies are required to confirm this finding given the premature termination of the trial.
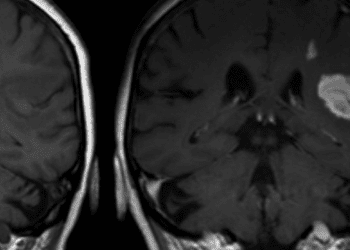
1. Exposure to high levels of ambient fine particulate matter over time is significantly associated with incidence of stroke.fine p
Evidence Rating Level: 1 (Good)
Ambient air pollution is a major public health concern in China and worldwide. Studies on short-term exposure to ambient fine particulate matter of diameter less than or equal to 2.5 mm (PM2.5) in China have reported increased risks of hospital admission for stroke. However, evidence of negative impacts of long-term exposure to air pollution is limited, especially in China, where ambient air pollution levels can be high. In this prospective cohort study, 117,575 individuals from the Prediction for Atherosclerotic Cardiovascular Disease Risk in China (China-PAR) project without stroke at baseline were studied to assess the association of long-term exposure to ambient PM2.5 and incidence of total, ischemic, and hemorrhagic stroke between 2000 and 2015. The China-PAR project was designed to investigate patterns of cardiovascular diseases and associated risk factors in the general Chinese population. At baseline, participants had a mean age of 50.9 years, 41.0% were men, and 23.9% were current smokers. Those with higher exposure to PM2.5 were less likely to be current smokers and had a higher BMI and systolic/diastolic blood pressures. During the study follow-up period, the average PM2.5 at residents’ residential addresses was 64.9 mg/m3 (range 31.2 to 97.0 mg/m3). Researchers found that during 900,214 person years of follow-up, there were 3,540 cases of incident stroke (ischemic: 63.0%; hemorrhagic: 27.5%). In both the age and sex adjusted model, and after multivariate adjustment, higher exposure to PM2.5 was significantly associated with an increased risk of stroke (p<0.001 for both). When participants were categorized according to quarters of exposure to PM2.5, researchers found that participants in the third (59.7 to 78.2 mg/m3) and fourth (78.3 to 97.0 mg/m3) levels had an increased risk of incident stroke (third: HR 1.30, 95% CI 1.13 to 1.49; fourth: HR 1.53, 95% CI 1.34 to 1.74), ischemic stroke (third: HR 1.49, 95% CI 1.25 to 1.77; fourth: HR 1.82, 95% CI 1.55 to 2.14) and hemorrhagic stroke (third: HR 1.40, 95% CI 1.07 to 1.82; fourth: HR 1.50, 95% CI 1.16 to 1.93) when compared to those in the first quarter (31.2 to 54.5 mg/m3). For each increase in 10 mg/m3 in PM2.5 concentration, the increased risks of incident stroke, ischemic stroke, and hemorrhagic stroke were 13% (HR 1.13, 95% CI 1.09 to 1.17), 20% (HR 1.20, 95% CI 1.15 to 1.25), and 12% (HR 1.12, 95% CI 1.05 to 1.20), respectively. Data on participants’ daily activity and location were not collected, which may have resulted in misclassification of exposure. Overall, this study suggests that long-term exposure to ambient PM2.5 at high concentrations may increase the risk of incident stroke.
Antiepileptic Drug Exposure in Infants of Breastfeeding Mothers With Epilepsy
- Antiepileptic drug exposure in infants of breastfeeding mothers being treated for epilepsy is low.
Evidence Rating Level: 2 (Good)
While breastfeeding for the first 6 months of life is currently recommended by the American Academy of Pediatrics, no consensus exists for the safety of breastfeeding when the mother is receiving antiepileptic drugs (AEDs). Previous studies have reported no adverse neurodevelopmental effects in infants breastfed by mothers taking AEDs, but no studies have measured blood concentrations of AEDs in mothers and infants to provide objective information on the extent of AED exposure via breastfeeding. In this prospective cohort study, 351 women and their 345 infants were studied to examine the percentage of infant-to-mother concentrations of AEDs using blood samples collected from infants who were breastfed and their mothers at the same visit between 5 and 20 weeks after birth. Of the 345 infants, 64.3% were breastfed after delivery, and 42.3% had AED concentrations available from a visit 5 to 20 weeks after births. Ultimately, 164 matching infant-mother concentrations were available for analysis from 135 mothers and 138 infants. The infant cohort had a median age of 13 weeks (range 5 to 20 weeks), and mothers had a median age of 32 years (range 18 to 47), and 87.4% of mothers were white. Most mothers (82.2%) were receiving monotherapy, and there was no difference in blood concentrations in infants who were breastfed from mothers receiving monotherapy versus polytherapy. Approximately half of the infants (49.3%) had AED concentrations less than the lower limits of quantification (LLoQ), and only infants exposed to lamotrigine, levetiracetam and zonisamide had AED concentrations over the LLoQ. The median percentage of infant-to-mother concentration for all 7 antiepileptic drugs and 1 metabolite (carbamazepine, carbamazepine-10,11-epoxide, levetiracetam, lamotrigine, oxcarbazepine, topiramate, valproate, and zonisamide) ranged from 0.3% (range 0.2% to 0.9%) to 44.2% (range, 35.2% to 125.3%). Using multiple linear regression models using data from 52 infant-mother pairs in which the mothers were taking lamotrigine, and 16 in which the mothers were taking levetiracetam, researchers found that only maternal lamotrigine concentrations were significantly associated with plasma lamotrigine concentrations in infants (Pearson correlation coefficient 0.58, p<0.001). Overall, this study indicates that AED drug exposure in infants being breastfed by mothers taking AED therapy is low. The infants in the study will be followed up until the age of 6 years to determine long-term outcomes.
1. In men with prostate cancer, functional outcomes following moderately hypofractionated radiation therapy are clinically equivalent to those following conventionally fractionated radiation therapy.
Evidence Rating Level: 2 (Good)
External-beam radiation therapy (RT) is a primary radical treatment option for men with localized or locally advanced prostate cancer. The conventionally fractionated RT regimen (C-RT) has traditionally been the standard of care, but a moderately hypofractionated regimen (H-RT) may offer a therapeutic and economic advantage by decreasing toxic effects. Several recent studies have demonstrated similar efficacy of C-RT and H-RT, but few have studied patient-reported outcomes, which may more reliably detect adverse treatment effects that are relevant to patients. In this retrospective cohort study, 17,058 men with prostate cancer underdoing either C-RT or H-RT were studied to compare patient-reported functional outcomes. Of the study cohort, 77% of participants responded, and 64.2% of those received C-RT. Men in the H-RT group were more likely to be older and have had pretreatment genitourinary procedures, and were less likely to have locally advanced disease and to have received androgen-deprivation therapy. Researchers found that H-RT was associated with statistically significantly higher Expanded Prostate Cancer Index Composite short-form 26 sexual scores (adjusted mean difference 3.3 points, 95% CI 2.1 to 4.5, p<0.001) and hormonal function scores (adjusted mean difference 3.2 points, 95% CI 1.8 to 4.6, p<0.001) than C-RT. However, these differences did not meet established thresholds for a clinically meaningful change. This study was limited by the lack of patient-reported outcomes immediately before treatment. Overall, the findings support recent guidelines that recommend H-RT as the standard of care for men with nonmetastatic prostate cancer.
Image: PD
©2020 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.