Age, bone mineral density may predict fracture after alendronate discontinuation
Image: PD
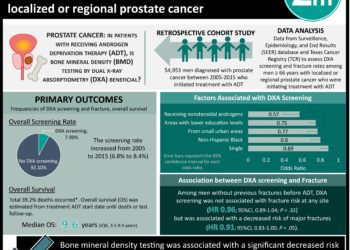
1. Older postmenopausal women with lower hip bone mineral density exhibit a greater risk of clinical fractures within 5 years of discontinuing alendronate therapy of 4 to 5 years duration.
2. Spine bone mineral density, serum bone-specific alkaline phosphatase (BAP), urine type 1 collagen cross-linked N-telopeptide (NTX), demographic factors (BMI and previous fractures) were not significantly associated with fracture risk.
Evidence Rating Level: 2 (Good)
Study Rundown: Bisphosphonate drugs like alendronate are effective at reducing the risk of fractures in patients with osteoporosis and low bone mineral density. However, recent concerns about the adverse effects and long-term safety of this therapy have lead many clinicians to consider temporary or permanent discontinuation of treatment. This post hoc analysis of patient data from the Fracture Intervention Trial Long-term Extension (FLEX) trial sought to link fracture risk in postmenopausal women who discontinued alendronate therapy after 4 or 5 years with specific diagnostic markers, including hip and spine bone mineral density (BMD) and biochemical markers of bone turnover.
The study found that within 5 years of discontinuing alendronate, 22% of study participants experienced 1 or more fractures. Older age and lower hip BMD at time of discontinuation were significantly associated with an increased risk of fractures. This study was limited by its relatively small sample size, imprecise measurements of bone turnover, limited follow-up, and its generalizability of results. Readers should also be aware this is a post hoc analysis, which is susceptible to type I error. However, based on these results, short-term monitoring of BMD and biochemical levels may not improve the identification of higher-risk individuals who should resume alendronate therapy.
Click to read the study in JAMA Internal Medicine
Click to read an accompanying editorial in JAMA Internal Medicine
In-Depth [post hoc analysis]: This post hoc analysis examined patient data from the placebo arm of the FLEX trial. The FLEX trial was a prospective, randomized trial of postmenopausal women between the ages of 61 and 86 who had been previously treated with 4 or 5 years of alendronate. Of the 1099 patients in the FLEX trial, 437 were assigned to the placebo arm in which they received daily placebo therapy instead of continuing on alendronate therapy.
During 5 years of follow-up in the FLEX trial, 82 of the 437 participants (22%) experienced 1 or more symptomatic fractures. Those that experienced a fracture were older (mean: 76.2 vs. 73.1, p < 0.001) and had lower total hip BMD (mean: 0.68 vs 0.73, p < 0.001) and femoral neck BMD (mean: 0.58 vs 0.62, p < 0.001) at time of alendronate discontinuation. The study found the relative risk of experiencing a fracture was 1.54 per 5 year increase of age (95% CI: 1.26 – 1.85) and 1.87 if total hip BMD levels were in the lowest tertile of measurements (95% CI: 1.20 – 2.92). Measurements of BAP and NTX, indicators of bone turnover, in the highest tertile were associated with a 1.39 and 1.33 relative risk of experiencing a fracture respectively, although neither figure was statistically significant.
More from this author: Oxytocin therapy improves sociocommunicational deficits in patients with autism, Intravitreal melphalan provides tumor control of vitreous retinoblastoma seeds, High volume geriatric trauma facilities provide better geriatric trauma outcomes, Laparoscopic Sleeve Gastrectomy does not reliably improve GERD symptoms,Active video games may improve pediatric weight management programs, Arterial stiffening associated with β-Amyloid deposition, Colorectal cancer resection associated with more complications in elderly
©2012-2014 2minutemedicine.com. All rights reserved. No works may be reproduced without expressed written consent from 2minutemedicine.com. Disclaimer: We present factual information directly from peer reviewed medical journals. No post should be construed as medical advice and is not intended as such by the authors, editors, staff or by 2minutemedicine.com. PLEASE SEE A HEALTHCARE PROVIDER IN YOUR AREA IF YOU SEEK MEDICAL ADVICE OF ANY SORT.