BNT162b2 SARS-CoV-2 vaccine is safe but less effective in cancer patients currently receiving treatment
1. After receiving the BNT162b2 SARS-CoV-2 vaccine, fewer cancer patients demonstrated seropositivity for COVID antibodies compared to age-matched healthy controls, but those that were seropositive had comparable titers to healthy controls.
2. The COVID vaccine was found to have an acceptable safety profile amongst cancer patients currently on treatment, with reported adverse events similar to the healthy population.
Evidence Rating Level: 2 (Good)
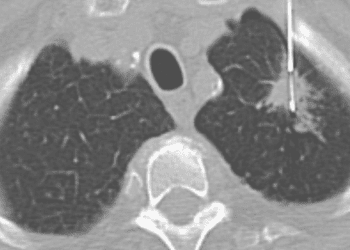
Study Rundown: Lung cancer may be a risk factor for severe COVID-19 infection. While cancer has not been an exclusion criteria in the BNT162b2 vaccine trials, cytotoxic therapy or systemic corticosteroid use was, effectively removing cancer patients from the trials used to approve these vaccines. There is not much data on efficacy and safety after real-world vaccine distribution to cancer patients. Despite this, guidelines currently recommend the vaccine for cancer patients of all types and treatments. This study prospectively measured serological status and safety outcomes of the SARS-CoV-2 BNT162b2 vaccine in patients with solid tumors and current anti-cancer treatments compared to an age-matched healthy control group. In- and out-patients with solid tumors receiving treatment and age-matched healthy controls after their first and second doses of COVID vaccine received serological testing. Whether these patients went on to get COVID was followed in hospital records. Fewer cancer patients above and below the age of 60 demonstrated seropositivity for COVID antibodies compared to healthy controls, but those that were seropositive had comparable titers. Age, sex, and cancer stage could not explain these differences. After a single dose of vaccine, more cancer patients were infected by COVID than control participants; however, after both doses, no COVID infection was detected in either group. Adverse events included injection site pain, warmness, redness, and swelling, fatigue, muscle/joint pain, headache occurred in cancer patients, similar to those documented in the healthy population. The older age of the cancer patient population compared to the best age-matched healthy control group presents a limitation since it is known that less immune response may be associated with older age. Another limitation is the lack of a control group of patients who were not vaccinated, especially since cancer patients are likely more adherent to COVID-19 safety precautions compared to the healthy control group to which they were compared on infection rates and titer. To further this, the “healthy control” group was mainly made of healthcare workers who qualified for the vaccine early, who were even more likely to be exposed to the coronavirus than the general population due to their work. There was also no systematic registration of corticosteroid use in this study which could have affected titer in the patient group. Finally, serologic titer has not yet been validated as a measurement of immunogenicity and remains just a surrogate. This study’s strengths are its early availability of data and clear significant differences.
Click to read the study in JAMA Oncology
Relevant Reading: COVID-19 in cancer patients: risk, clinical features, and management
In-Depth [prospective cohort]: This study included patients with solid tumors receiving intravenous infusion at an ambulatory clinic or inpatient clinic at Rambam Health Care Campus (RHCC), Haifa, Israel (n=232, 57% men, age = 66±12.09 years) and a cohort of age-matched healthy controls (n=261, 45% men, age = 59±15.7 years) mainly comprised of healthcare workers. At least 10 days after their first dose and/or at 14 days after their second dose of COVID-19 vaccine, serological tests were performed to measure anti-SARS-CoV2 antibody titer (seropositive was defined as >15 U/mL); if seronegative, a blood sample was taken for complete blood cell count, liver enzyme levels, and creatinine levels. Hospital records were accessed to identify reverse transcriptase polymerase chain reaction assay-detected COVID-19 infection. 74% of the cancer group had metastatic cancers. The cancers in this study were mainly gastrointestinal (27%), genitourinary (21%), lung (19%), or breast (18%). Most cancer patients were on chemotherapy (58%), biologics (35%), or immunotherapy (36%), with some on more than one. Fewer cancer patients over the age of 60 had positive serologic testing compared to control (30% vs 80%, P < .001). The titer did not differ for seropositive cancer patients compared to seropositive control (40.25 U/mL vs 43.5 U/mL). Similarly, fewer cancer patients under the age of 60 demonstrated seropositive results compared to control (27% vs 94%, P < .001), with no difference in titer (72.5 vs 74.0). There was no significant difference between cancer patient’s seropositive rates by age, sex, or disease stage. An adjusted Poisson regression model predicts that after the first dose 12.5 patients would be expected to be seropositive per 1000 person-days (P < .001; 95% CI, 3.4-45.7) compared with 48.5 control participants (P < .001; 95% CI, 37.2-63.2). There was lower incidence of seropositivity in the cancer group compared to the control group (26.5% vs 84.3%, P < .001). After 1 dose of vaccine there was more cases of COVID infection in the cancer patient group compared to the control group (2 cases vs 0 cases); no cases of COVID infection were identified after two doses of vaccine in either group. Cancer patients reported side-effects of the vaccine including injection site pain (69%), warmness (9%), redness (8%), and swelling (4%). Reported systemic side effects in cancer patients included fatigue (24%), muscle/joint pain (13%), headache (10%), and fever (1%). 28% had 50% higher liver enzymes and 5% of patients had new lymphadenopathy. These side effects are comparable to rates reported elsewhere for the general population.
Image: PD
©2021 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.

![2MM: AI Roundup- AI Cancer Test, Smarter Hospitals, Faster Drug Discovery, and Mental Health Tech [May 2nd, 2025]](https://www.2minutemedicine.com/wp-content/uploads/2025/05/Untitled-design-350x250.png)