Crizanlizumab use lowers rates of sickle cell crises
1. Patients with sickle cell disease treated with high-dose crizanlizumab experienced lower annualized rates of sickle cell crisis compared to placebo treatment, regardless of their baseline use of hydroxyurea.
2. Those treated with high-dose crizanlizumab remained without a sickle cell crisis on average significantly longer into the study period than those treated with placebo.
Evidence Rating: 1 (Excellent)
Study Rundown: The pathogenesis of sickle cell disease has been reported to be based on vaso-occlusive events which increases tissue inflammation. In addition to damaging tissue, these occlusive events are reported to be extremely painful and contribute to increased mortality risk amongst those with sickle cell disease. Adherence of erythrocytes and leukocytes to vascular endothelium is a fundamental aspect of vaso-occlusive events, and P-selectin expression by endothelium has been shown to promote adherence. This study aimed to evaluate the effects of crizanlizumab, an antibody against P-selectin, on reducing the rate of a sickle cell crisis.
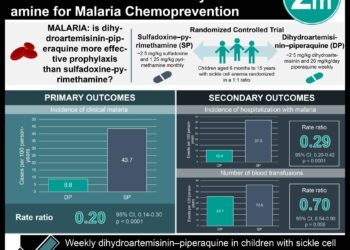
People with sickle cell disease, including those on baseline hydroxyurea or not, who had experienced recent sickle cell-related pain crisis were included in the trial. Patients were randomized on a 1:1:1 fashion into groups receiving high-dose crizanlizumab, low-dose crizanlizumab, or a placebo. Treatments were administered for 52 weeks, and patients were followed to assess for occurrence of sickle cell-related pain crisis, time from study initiation to sickle cell-related pain crisis, and other markers of treatment efficacy and safety. Those treated with high-dose crizanlizumab experienced significantly fewer pain crises per year than those in the placebo group. A first sickle cell-related pain crisis during the study period occurred later on average for those in the high-dose crizanlizumab group compared to placebo.
Click to read the study, published today in NEJM
Relevant Reading: Inhibition of cell adhesion by anti–P-selectin aptamer: a new potential therapeutic agent for sickle cell disease
In-Depth [randomized controlled trial]: From 2013 to 2015, 198 patients at 60 international sites were randomly assigned to receive high-dose crizanlizumab (n = 67), low-dose crizanlizumab (n = 66), or placebo (n = 65). Patients included in the study had sickle cell disease, were 16-65 years old, had 2-10 sickle crises in the past year, and could be on hydroxyurea or not. Those being chronically transfused were not included. Randomization to treatment group was stratified by number of crises in the prior year (2-4 or 5-10) and hydroxyurea use (yes or no). The study included a 4 week loading phase, 52-week treatment phase with treatment administered every 4 weeks, and an evaluation phase 6 weeks after conclusion of treatments. Assessed via intention-to-treat, those in the high-dose group experienced 45.3% fewer crises per year than those in the placebo group (rate of crisis 1.63 vs. 2.98, p = 0.01). A per-protocol analysis of participants in the high-dose group demonstrated a 52.3% fewer crises per year than the placebo group (rate of crisis 1.04 vs. 2.18, p = 0.02). The low-dose group was not significantly different from the placebo group in either of the prior analyses. Patients treated with or without hydroxyurea experienced fewer crisis per year in the high-dose group compared to the low dose group, though the difference was not statistically significant (rate of crisis with hydroxyurea 2.43 vs. 3.58; rate of crisis without hydroxyurea 1.00 vs. 2.00). The median time to first crisis after starting treatment was longer in the high-dose group compared to those treated with placebo (rate of crisis 4.07 vs 1.38 months, p = 0.001). Serious adverse events such as headache, back pain, nausea, arthralgia were reported in 55 participants and was not significantly higher in one study group over the others. No detectable antibody response against crizanlizumab was detected in any patient during the trial.
Image: CC/Wiki
©2017 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.