Device that increases coronary sinus pressure aids refractory chest pain
1. For patients with refractory chest pain (angina) and who are not candidates for revascularization, a new device that increases coronary sinus pressure provides significant symptomatic benefit.
Evidence Rating Level: 1 (Excellent)
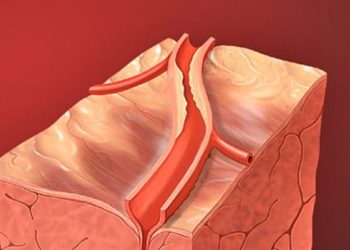
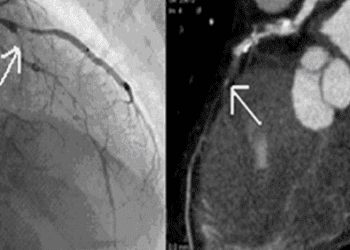
Study Rundown: Medical therapy is currently the only option for patients with refractory angina and deemed not candidates for revascularization. Recent studies have focused on a new device that is implanted into and narrows the coronary sinus to increase pressure and improve myocardial perfusion by theoretically recruiting collateral circulation. The process of implanting these devices is less invasive than revascularization, thus providing a lower risk method of improving symptoms and quality of life for patients with refractory angina.
This phase two, double-blinded, multicenter trial randomized 104 patients with moderate to severe angina [Canadian Cardiovascular Society (CCS) class 3 and 4] to either therapy with the new device or sham procedure. By follow up at 6 months, the primary endpoint of a greater than two CCS class improvement was achieved by 35% with the implanted device and 15% in the control group. The rate of adverse events in both groups were also not statistically significant. In both groups, there was no significant difference in exertional capacity or improvement in left ventricular function, although this study was underpowered for these secondary endpoints.
These results show promise for a device-based therapy for patients with refractory angina who are not candidates for revascularization; an expanding demographic given the aging population and prevalence of ischemic heart disease. While this device is not a cure, it may provide benefit in terms of quality of life. Future studies are likely to enroll more patients to study the long-term efficacy (including device migration or occlusion) and safety of these coronary sinus devices.
Click to read the study, published today in NEJM
Click to read an accompanying editorial in NEJM
In-Depth [randomized controlled trial]: 104 patients with angina (CCS class 3 or 4) were randomized 1:1 to either procedure for coronary sinus device or sham procedure to a primary endpoint of a greater than two class improvement in CCS class by 6 months (35% versus 15%, device versus control, p=0.02). Baseline characteristics were well-matched in both groups including comorbidities, left ventricular ejection fraction, and number of anti-anginal medications. At 6 months, 35% of patients in the treatment group had an improvement of at least two CCS classes, as compared to 15% in the control group (P=0.02). In addition, for 71% of patients in the treatment group, the device was associated with improvement of at least one CCS angina class, as compared to 42% in the control group (P=0.003).
All patients were treated with dual anti-platelet therapy for the duration of this study. One adverse event was reported in 64% treated with device and 69% with sham procedure (p=0.68). There was one peri-procedural myocardial infarction in the device-treated group versus three myocardial infarctions in the control group.
More from this author: Factor XI anti-sense oligonucleotide bests enoxaparin for venous thromboembolism in knee surgery, Rituximab superior to azathioprine in autoimmune vasculitis, Ultrasound as effective as CT at identifying high-risk kidney stones, reduces radiation exposure, Lower blood transfusion threshold in septic shock safe at 90-days, Influenza vaccination in pregnancy protects both mother and infant, Sirolimus may prevent graft failure in patients with renal transplantation for antiphospholipid syndrome
Image: PD
©2015 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. No article should be construed as medical advice and is not intended as such by the authors, editors, staff or by 2 Minute Medicine, Inc.