Enfortumab vedotin prolongs survival in previously treated advanced urothelial carcinoma
1. Enfortumab vedotin showed improved overall survival when compared to chemotherapy in patients with previously treated advanced urothelial carcinoma.
2. The incidence of serious adverse events was similar between both groups.
Evidence Rating Level: 1 (Excellent)
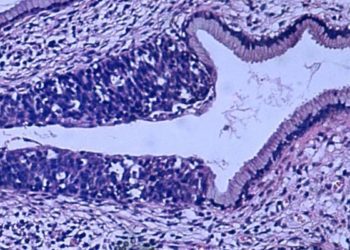
Study Rundown: In advanced urothelial carcinoma, platinum-based chemotherapy and immunotherapy are the first and second line therapies. The optimal regiment after progression is unclear. Enfortumab vedotin is an antibody directed against nectin-4, which has high expression in urothelial carcinoma and is thought to be a contributor to cancer growth. In this phase 3, open-label clinical trial, for patients with advanced carcinoma who previously progressed on chemotherapy and PD-1/PD-L1 inhibitor, patients were either randomized to enfortumab vedotin or standard chemotherapy. Patients treated with enfortumab vedotin lived longer compared to standard chemotherapy and had better overall response rates. High-grade adverse events were similar among both groups; the most common being maculopapular rash, decreased neutrophil counts, and fatigue in the enfortumab vedotin group. However, it is unclear how enfortumab vedotin compares to FGFR inhibitors in tumors with a susceptible mutation. Overall, this trial demonstrated the superior effect of enfortumab vedotin in the treatment of refractory urothelial carcinoma when compared to standard single-agent chemotherapy.
Click here to read the study in the NEJM
Relevant Reading: Enfortumab Vedotin Antibody–Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models
In-Depth [randomized controlled trial]: This was a phase three, randomized, open-label trial of 608 patients randomized to either enfortumab vedotin or single-agent standard chemotherapy (docetaxel, paclitaxel, or vinflunine). The primary endpoint was overall survival. 134 deaths occurred in the enfortumab vedotin group (median survival, 12.88 months) and 167 deaths (median survival, 8.97 months) occurred in the chemotherapy group. The hazard ratio (HR) for death was 0.70 (95% confidence interval [CI] 0.56 to 0.89, P = 0.001). Progression-free survival showed the same trend of superiority for enfortumab vedotin. The overall response rate was 40.6% in the enfortumab vedotin group (95% CI, 34.9 to 46.5) and 17.9% (95% CI, 13.7 to 22.8) in the chemotherapy group (p < 0.001). Adverse events were common and similar between both groups (enfortumab vedotin group, 93.8%; chemotherapy group, 91.8%). The most frequent grade 3 or higher events in the enfortumab vedotin group was maculopapular rash (7.4%), fatigue (6.4%), and decreased neutrophil count (6.1%). The most frequent grade 3 or higher events in the chemotherapy group were decreased neutrophil count (13.4%), anemia (7.6%), decreased white cell count (6.9%), neutropenia (6.2%), and febrile neutropenia (5.5%). Overall, while the safety profile of the enfortumab vedotin group does pose some caution, the efficacy of this regiment is superior to standard chemotherapy.
Image: PD
©2021 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.