Ibrutinib effective for previously treated Waldenström’s macroglobulinemia
1. Ibrutinib, an inhibitor of Bruton’s tyrosine kinase, produced a response in 90.5% of patients with Waldenström’s macroglobulinemia.
2. Patient responses to ibrutinib were influenced by the presence of two mutations, MYD88L265P and CXCR4WHIM.
Evidence Rating Level: 2 (Good)
Study Rundown: Waldenström’s macroglobulinemia is a disease of B cells, the antibody-producing cells of the immune system. Ibrutinib is a small molecule that inhibits Bruton’s tyrosine kinase (BTK), an enzyme that plays a role in the maturation of B cells. MYD88L265P, a mutation found in patients with Waldenström’s macroglobulinemia, works by altering BTK activity. As such, it is unsurprising that ibrutinib is active against cells with this mutation. However, Waldenström’s macroglobulinemia cells often also carry mutations in CXCR4 which can make them resistant to ibrutinib.
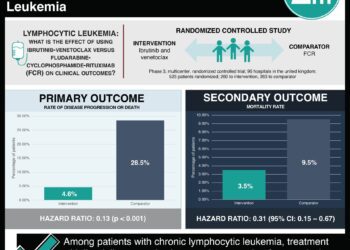
In this study, 63 patients with Waldenström’s macroglobulinemia, who had received previous treatment, were treated with ibrutinib. The authors evaluated the response to ibrutinib by genetic profile of the Waldenström’s cells. Patients with the MYD88L265P mutation and normal CXCR4 had a 100% overall response rate and a 91.2% major response rate. Patients with both mutations had a lower response rate (85.7% and 61.9%), and patients with neither mutation had the lowest response rates (71.4% and 28.6%), though there was no significant difference in the response rates between these two groups.
The study’s conclusions are limited by the absence of a control group. However, the use of previously-treated patients suggests that ibrutinib did alter their disease course, and these response rates compare favorably against those reported for other therapies.
Click to read the study, published today in NEJM
Relevant Reading: Bruton Tyrosine Kinase Inhibitor Ibrutinib (PCI-32765) Has Significant Activity in Patients With Relapsed/Refractory B-Cell Malignancies
In-Depth [prospective cohort]: This multicenter cohort study assembled 63 patients over 3 sites in the US and treated them all with ibrutinib for twenty-six 4-week cycles until disease progressed or severe toxicity developed.
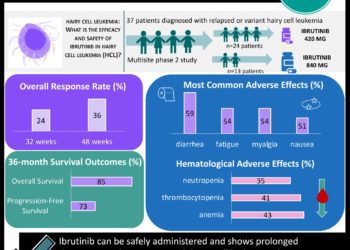
All patients had been previously treated for their disease, and 40% of patients had disease that was resistant to their last treatment. Overall, ibrutinib produced a 90.5% response rate and a 73% major response rate. Response to treatment was measured by the decrease in serum IgM levels, and a major response was defined as a fall in IgM levels of >=50% or a complete response.
The most common side effect was neutropenia (14 patients), followed by thrombocytopenia (9 patients). Severe, potentially life-threatening hematologic side effects did develop in 6 patients. Patients with more intensive prior treatment were more likely to develop serious side effects.
Image: CC/Blausen.com Staff
©2015 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.